
Paper: Mathew R, et al. Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock. N Engl J Med. 2021 Aug 5. PMID: 34347952
Clinical Question: What is the efficacy and safety of Milrinone compared to Dobutamine in patients with cardiogenic shock?
What They Did:
- Dobutamine Compared with Milrinone (DOREMI) Trial
- Randomized double-blind trial performed at a single quaternary care center
- Computer-generated random sequence was stratified according to affected ventricle (left or both vs right ventricle)
- Upon enrollment, patient’s cardiogenic shock was characterized according to the Society for Cardiovascular Angiography and Intervention (SCAI) definitions of A, B, C, D, E (see Figure 1). The majority of patients (≈80%) in this study were Class C.
- Patients were assigned to one of two groups with the following standard dosing scale:
- Dobutamine group: Dosing ranged from stage 1 to stage 5 which corresponded to 2.5, 5.0, 7.5, 10.0 and >10ug/kg/min
- Milrinone group: Dosing range of 0.125, 0.250, 0.375, 0.500 and >0.500ug/kg/min
- Adjustment of the doses according to stage was performed by a blinded treating team based on clinical judgement
- If at any time the assigned therapy was considered unsafe, the treating physician was unblinded and treated the participant with an open-label inotrope
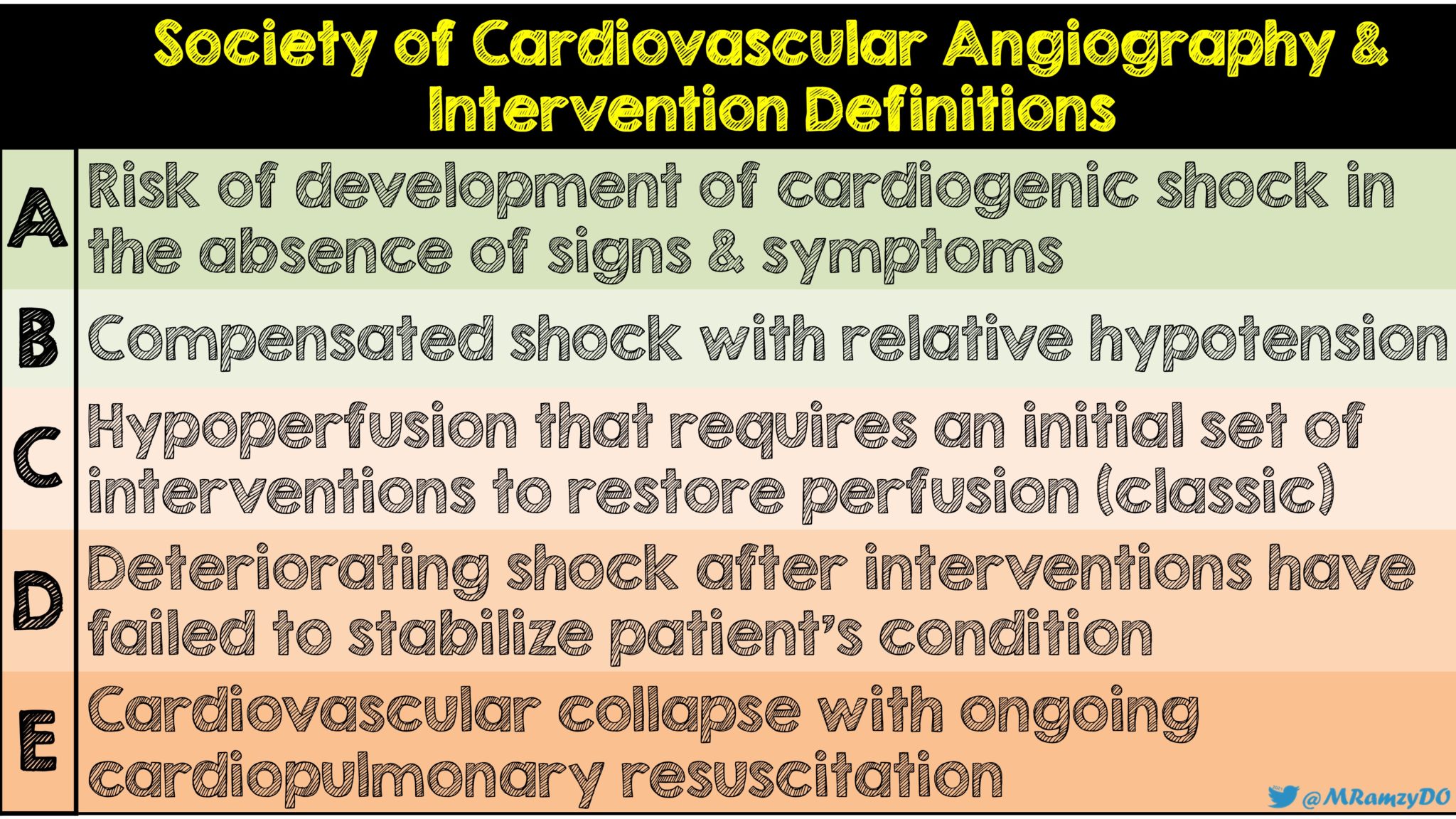
Figure 1: Society for Cardiovascular Angiography and Intervention definitions (Image by: @MRamzyDO)
Inclusion Criteria:
- Patients 18 years or older admitted to the cardiac ICU and have had one of the following indications for inotropic therapy:
- Diagnosis of cardiogenic shock and a systolic blood pressure of < 90mmHg with end-organ dysfunction
- Clinical evidence of systemic and/or pulmonary congestion despite vasodilator and/or diuretic use
- Acute coronary syndrome complicated by cardiogenic shock with hemodynamic reduction in cardiac index
- Clinically determined need to improve cardiac output in addition to vasopressor therapy
- Treating team’s discretion that inotropic therapy is needed for developing cardiogenic shock without current evidence of hypoperfusion
Exclusion Criteria:
- Presented with out-of-hospital cardiac arrest
- Pregnant patients
- Already had milrinone or dobutamine started prior to randomization
- Discretion of treating physician that patient was not eligible for the study
- Participation in another interventional trial
- Inability to obtain written informed consent from patient or designated decision maker
Outcomes:
Primary
- Composite of the following:
- In-hospital death from any cause
- Resuscitated cardiac arrest
- Receipt of a cardiac transplant or mechanical circulatory support
- Non-fatal myocardial infarction
- Transient ischemic attack or stroke diagnosed by a neurologist
- Initiation of renal replacement therapy
Secondary
- Efficacy Endpoints
- Total Time on Inotropes
- Length of stay in CCU greater than or equal to 7 days
- Need for and total number of days requiring non-invasive and invasive mechanical ventilation
- Change in cardiac index and/or pulmonary artery pressure
- Presence of acute kidney injury
- Normalization of serum lactate
- Arrhythmia requiring medical team intervention
- Safety Endpoints:
- Sustained hypotension
- Ventricular or Atrial arrhythmias
- Need for IV or oral anti-arrhythmics
- Need for up-titration or addition of new vasopressor therapy
Results:
- A total of 319 patients were screened and of them 192 were enrolled, 96 in each treatment group.
- At randomization, 10 patients had an intraaortic balloon pump in place and 23 had a pulmonary artery catheter
- Median serum lactate in both groups was 2.9 mmol/L indicating a similar amount of hypoperfusion between the two groups
Critical Results:
- There was so significant difference between the groups in the following individual components of the primary outcomes:
- In-hospital death from any cause
- Occurrence of resuscitated cardiac arrest
- Receipt of mechanical circulatory support
- Occurrence of transient ischemic attack or stroke diagnosed by a neurologist
- Initiation of renal replacement therapy
- No participant underwent cardiac transplantation and only one non-fatal myocardial infarction occurred
- There was no significant difference between the groups in any of the secondary outcomes
- Overall, there was no significant differences between the treatment groups in heart rate, mean arterial pressure, serum lactate, serum creatinine, or hourly urine output
Strengths:
- Helps provide additional information regarding a clinically relevant topic where a knowledge gap exists in the management of cardiogenic shock
- Independent data and safety monitoring board regularly monitored enrollment and safety data
- Medication bags and IV pump screens were concealed to ensure blinding
- Performed a post hoc sensitivity analysis of the primary outcome for adjustment of baseline invasive mechanical ventilation, previous MI, previous PCI and vasopressor use before randomization
- Inclusion criteria were based predominantly on clinical assessment in an attempt to improve external validity
- Designed to include a broad range of patients in the phases of shock that are treated with inotropes
- Dosing adjustments were performed by a blinded treating team to help reduce bias
- Re-evaluated the clinical significance of ICU length of stay as a primary outcome relative to other variable in the composite outcome and made it a secondary outcome
- Had very well thought out secondary outcomes divided into efficacy and safety
- Empowered treating physicians to unblind assignment and treat with open label if therapy was considered too unsafe to continue
Limitations:
- Only in-hospital outcomes were evaluated and this was an ICU-only study
- Both groups were not well balanced as there was more previous MI and PCI in the milrinone group. Additionally time from randomization to ICU admission was longer in the milrinone group
- Single center limits external generalizability
- Overall small sample size of patients
- Primary outcome is a composite of multiple variables which introduces risk and not as clinically useful as the individual components
- No follow-up after index hospitalization
- Trial was underpowered to detect smaller differences and had even less power for the comparison of the different components of the primary outcome
- Dose adjustments were based on individual physician discretion rather than standardized protocol guided by biochemical or hemodynamic measures. Differences in dose adjustments and resuscitation could have further contributed to unbalance between the groups
Discussion:
- This is the largest study to date, comparing two specific drugs used to manage cardiogenic shock
- The authors hypothesized there would be a whopping 20% difference in the primary outcome between the medications. This large difference can be seen in the wide confidence intervals in the primary outcome. Therefore, smaller differences cannot be commented on but could be clinically significant.
- Most patients were SCAI class C or D (class or deteriorating cardiogenic shock). This study does not answer questions on more aggressive treatments in earlier phases of cardiogenic shock (ie. class B). Earlier treatment could possibly alter the course of cardiogenic shock and potentially even improve outcomes, however this latter point is outside the scope of this trial and requires further study
- A small number of patients had pulmonary artery catheters and therefore no conclusions can be made about the benefits or harms of this intervention in this patient population based on this study
- The authors made it a point to make the inclusion criteria driven by clinical assessment as opposed to protocol based on biochemical or hemodynamic measures. This has the benefit of resembling clinical variable and expanding external validity at the cost of introducing bias
- The dose adjustments were made per physician discretion. Although this closely mirrors clinical practice, it allows for potential differences in overall resuscitation of the patient and between the treatment groups.
- Composite outcomes have pros and cons. While they can help statistical efficiency and reduce the sample size needed to see a particular effect, they also group together multiple endpoints of variable importance. Such is the case in this trial where in-hospital death from any cause does not hold the same weight as initiation of renal-replacement therapy.
- Another factor to consider with the use of a composite outcome, is endpoint selection. Certain variables can be cherry-picked before or after data analysis as part of statistical sleight of hand. The authors did not explicitly comment on why they chose 6 endpoints that made up their primary composite outcome.
- One last comment on composite outcomes relates to their practical clinical utility. Due to the combined nature of the variables and their weight of importance, it is difficult to ascertain how clinically applicable one will be over the other in one specific patient.
- The authors’ use of a post hoc sensitivity analysis of the primary outcome for certain characteristics is important because there were some potentially clinically important and unbalanced baseline characteristics between the two groups.
- A major limitation to this study is that only in-hospital outcomes were evaluated on a single index hospitalization. Since there was no follow-up, any differences in outcomes beyond that hospitalization were not assessed.
- Regardless of which medication was used, it’s important to note the high incidence of in-hospital death across both groups. This was also seen in previous studies.2
- Interestingly, there was no higher incidence of any particular adverse events with one drug over the other. Therefore, prior to administering either, physicians should consider other variables such as availability, cost, nursing familiarity and any other features in the patient’s clinical condition when choosing milrinone or dobutamine.
Author’s Conclusion:
- In patients with cardiogenic shock, no significant difference between milrinone and dobutamine was found with respect to the primary composite outcome or important secondary outcomes.
Our Conclusion:
- We agree with the author’s conclusion that there is no significant difference between milrinone and dobutamine when treating patients in cardiogenic shock. However, due to the use of a composite outcome, limited external validity and the majority of patients with classic or deteriorating cardiogenic shock (SCAI class C and D respectively), the results of this single-center trial with small sample size should be interpreted with caution. The decision to use one drug over the other should be made at the discretion of the treating physician based on the patient’s overall clinical picture.
Clinical Bottom Line:
- There was no significant difference in the primary composite outcome between milrinone and dobutamine in patients with classic or deteriorating cardiogenic shock in this single-center trial with a small sample size. The lack of difference found in this trial could simply be because initiation of therapy may have been too late in the clinical course of illness as is seen by the high mortality rates in this population across both groups. The decision on which drug to use should be tailored to institutional availability, cost, nursing familiarity, the individual patient and their clinical condition.
REFERENCES:
- Mathew R, et al. Milrinone as Compared with Dobutamine in the Treatment of Cardiogenic Shock. N Engl J Med. 2021 Aug 5. PMID: 34347952
- Hochman JS, et al. Early revascularization in acute myocardial infarction complicated by cardiogenic shock. SHOCK Investigators. Should We Emergently Revascularize Occluded Coronaries for Cardiogenic Shock. N Engl J Med. 1999 Aug 26; PMID: 10460813
For More Thoughts on This Topic Checkout:
- The Bottom Line: DOREMI
Post Peer Reviewed By: Salim Rezaie, MD (Twitter: @srrezaie)
The post DoReMi Trial: Milrinone vs Dobutamine for Treatment of Cardiogenic Shock appeared first on REBEL EM - Emergency Medicine Blog.
