

REBEL Cast Ep97: The NoPAC Trial – TXA for Epistaxis?
Article: Reuben A et al. The Use of Tranexamic Acid to Reduce the Need for Nasal Packing in Epistaxis (NoPAC): Randomized Controlled Trial. Ann Emerg Med 2021 Link
Clinical Question: Does the use of topical, intranasal TXA reduce the need for application of anterior nasal packing in ED patients with epistaxis who fail conservative management?
Population: Patients > 18 years of age presenting with persistent epistaxis after local pressure was applied.
Intervention: Tranexamic acid (TXA) 200 mg in 4 ml applied to a cotton wool dental roll (could be repeated X 1). Dental roll held in place for 10 minutes with pressure
Control: Cotton wool dental roll soaked in sterile water. Dental roll held in place for 10 minutes with pressure
Outcomes:
- (Primary): Use of anterior nasal packing (of any type) during the index ED visit regardless of any other additional treatments (intention to treat analysis)
- (Secondary): Hospital admission, need for blood transfusion, recurrent epistaxis, thrombotic events, hospital reattendance within 1 week.
Design: Randomized, double-blind, parallel-group, placebo-controlled trial in 26 centers in the UK
Excluded: Hemodynamic instability, epistaxis secondary to trauma, out-of-hospital packing placed, documented allergy to TXA, “expected” by the ear, nose and throat in-patient team for specialist treatment, known/suspected nasopharyngeal malignancy, pregnancy, hemophilia, inability to provide consent.
Primary Results:
- Patients enrolled
- n = 496 patients randomized
- TXA: n = 254 (51.2%)
- Placebo: n = 242 (48.8%)
- n = 834 patients excluded as a research nurse was unavailable
- Anticoagulant use
- TXA: 61%
- Placebo: 68.6%
- Follow up data (either by telephone call or hospital records) available for 100% of patients
Critical Findings:
- Per-protocol analysis
- Excluded participants who did not have second dose of treatment when indicated
- Negative for primary outcome
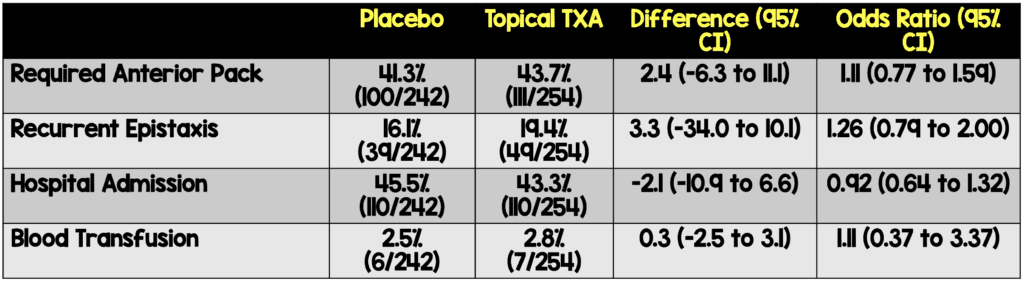
Strengths:
- Study asks a clinically relevant question with a patient centered outcome
- Largest study to investigate the role of topical intranasal TXA as an adjunct to standard therapy
- Randomization was appropriately performed
- Double-blinded
- Surpassed the minimum number of patients required for their power calculation
- The primary outcome was evaluated in 100% of patients
Limitations:
- Convenience sample of patients
- Estimated that 95% of patients would require anterior packing for the power calculation but number was far less. The study may have been underpowered to find a difference
- Did not exclude those with posterior epistaxis which has been seen in prior studies
Discussion:
- Key Point: This is not a study of all comers with epistaxis
- All patients had local pressure applied followed by a topical vasoconstrictor (phenylephrine) prior to receiving either TXA or placebo.
- Only after failure of simple first aid (i.e. direct pressure) and application of topical vasoconstrictor failed were patients enrolled into the study
- In the US, the most common vasoconstrictor is oxymetazoline. It is unclear if one agent is superior to the other and thus, it’s unclear how this effects application of this evidence. However, all patients had continued bleeding after application leading to further care so the impact is likely minimal.
- Prior studies have used higher doses (500 mg) of TXA. However, it’s unclear if the larger amount of TXA that the applicator was soaked in resulted in a larger amount of TXA reaching the mucosal tissue due to absorption by the applicator
- Investigators assumed that the majority (95%) of patients would require anterior packing. However, the number was considerably lower (42.5%)
- As a result, study may have been underpowered to find a difference
- However, there wasn’t even a hint of benefit to TXA in this trial
- Endpoint of this study (requiring anterior packing) was different than what was seen in prior studies (stopping bleeding). We assume the packing was placed because bleeding was not controlled but it’s possible there were other indications
- Admission rate (~44%) is quite high in this study and not reflective of care in other countries. However, discussion with the authors reveals that this is likely due to the UK “4-hour rule”
- Prior Studies
- Zahed 2013: Topical TXA superior to anterior packing
- Randomized but unblinded
- Unclear what care was provided prior to the intervention
- Used 500 mg of TXA
- Zahed 2017 : Topical TXA superior to anterior packing in patients on antiplatelet agents
- Randomized but unblinded
- Unclear what care was provided prior to the intervention
- Used 500 mg of TXA
- Small study with 62 patients in each arm
- Akkan 2019 : Topical TXA had similar efficacy to anterior packing but was better than compression with saline
- Randomized but unblinded
- Unclear what care was provided prior to the intervention
- Used 500 mg of TXA
- Small study with 45 patients in each arm
- This is the largest trial and only double-blind study looking at topical TXA for epistaxis.
- Zahed 2013: Topical TXA superior to anterior packing
Based on the results of the NoPAC trial…I will still use topical TXA in the management of anterior epistaxis?
— Salim R. Rezaie, MD (@srrezaie) March 6, 2021
Authors Conclusions: “In patients presenting to an ED with atraumatic epistaxis that is uncontrolled with simple first aid measures, topical tranexamic acid applied in the bleeding nostril on a cotton wool dental roll is no more effective than placebo at controlling bleeding and reducing the need for anterior nasal packing.”
Our Conclusions: We agree with the authors. In this well-done RCT, TXA was not more effective than placebo for reducing the need for anterior nasal packing in patients with epistaxis.
Potential to Impact Current Practice: This is the largest and most methodologically rigorous study to date on the use of topical TXA in epistaxis. When considering all the literature, this study definitely swings the pendulum towards advising against routine use. However, it is not clear based on all the available evidence exactly what is the best approach leaving ample room for clinical preference.
For More on This Topic Checkout:
- REBEL EM: Topical TXA in Epistaxis
- FOAMCast: Tranexamic Acid in Epistaxis
- First10EM: NoPAC – No Benefit from TXA in Epistaxis
- St. Emlyn’s: The NoPAC Trial – TXA Does not Work for Epistaxis
- The SGEM: SGEM #321 – The Times they are a Changin’ for TXA in Epistaxis?
References:
- Gifford TO, Orlandi RR. Epistaxis. Otolaryngol Clin North Am. 2008;41:525-536. PMID: 18435996
- Pallin DJ et al. Epidemiology of epistaxis in US emergency departments, 1992 to 2001. Ann Emerg Med. 2005;46:77-81. PMID: 15988431
- Zahed R et al. A new and rapid method for epistaxis treatment using injectable form of tranexamic acid topically: a randomized controlled trial. Am J Emerg Med 2013; 31: 1389-92. PMID: 23911102
- Zahed R et al. Topical Tranexamic Acid Compared With Anterior Nasal Packing for Treatment of Epistaxis in Patients Taking Antiplatelet Drugs: Randomized Controlled Trial. Acad Emerg Med 2017. PMID: 29125679
- Akkan S et al. Evaluating Effectiveness of Nasal Compression With Tranexamic Acid Compared With Simple Nasal Compression and Merocel Packing: A Randomized Controlled Trial. Ann Emerg Med 2019; 74(1): 72-78. PMID: 31080025
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post REBEL Cast Ep97: The NoPAC Trial – TXA for Epistaxis? appeared first on REBEL EM - Emergency Medicine Blog.
