
Paper: Hughes CG et al. Dexmedetomidine or Propofol for Sedation in Mechanically Ventilated Adults with Sepsis. NEJM 2021. PMID: 33528922 [Access on Read by QxMD]
Clinical Question: In adult patients with sepsis, acute respiratory failure, and requiring mechanical ventilation, is dexmedetomidine or propofol superior in reducing neurological dysfunction (i.e delirium or coma) during a 14 day intervention period?
What They Did:
- Maximizing the Efficacy of Sedation and Reducing Neurological Dysfunction and Mortality in Septic Patients with Acute Respiratory Failure (MENDS2)
- Multicenter, double-blind randomized controlled trial of adult patients mechanically ventilated with sepsis at 13 medical centers in the US
- Randomized to:
- Dexmedetomidine 0.2 to 1.5ug/kg/hr
- Propofol 5 to 50ug/kg/min
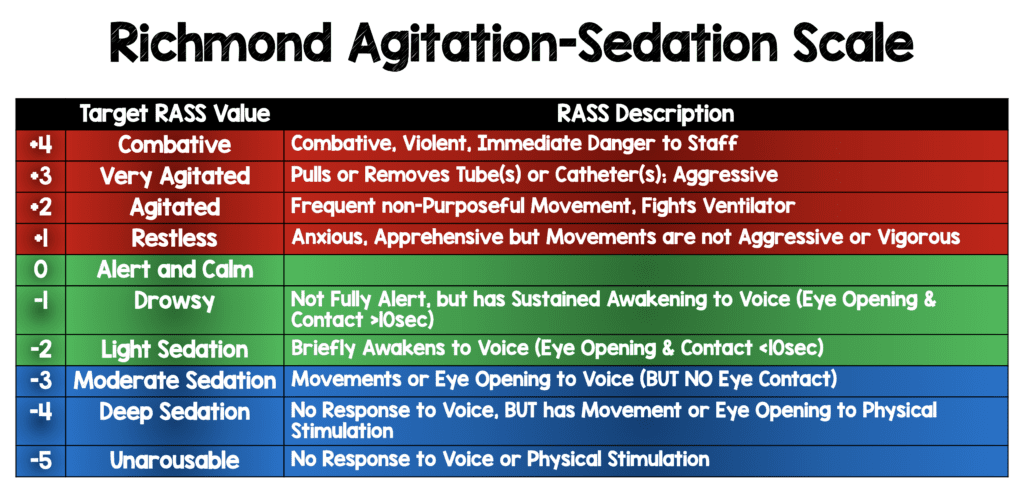
- Doses adjusted every 10 minutes to achieve target sedation goals according to the Richmond Agitation-Sedation Scale (RASS)
- RASS scores range from -5 (unresponsive) to +4 (combative)
- Target RASS score was 0 to -2
-
- Pain treated with intermittent opioid boluses or fentanyl infusions
- Confusion Assessment Method for the ICU (CAM-ICU) was used for assessment of delirium
- Critical Care Pain Observation Tool was used for assessment of pain
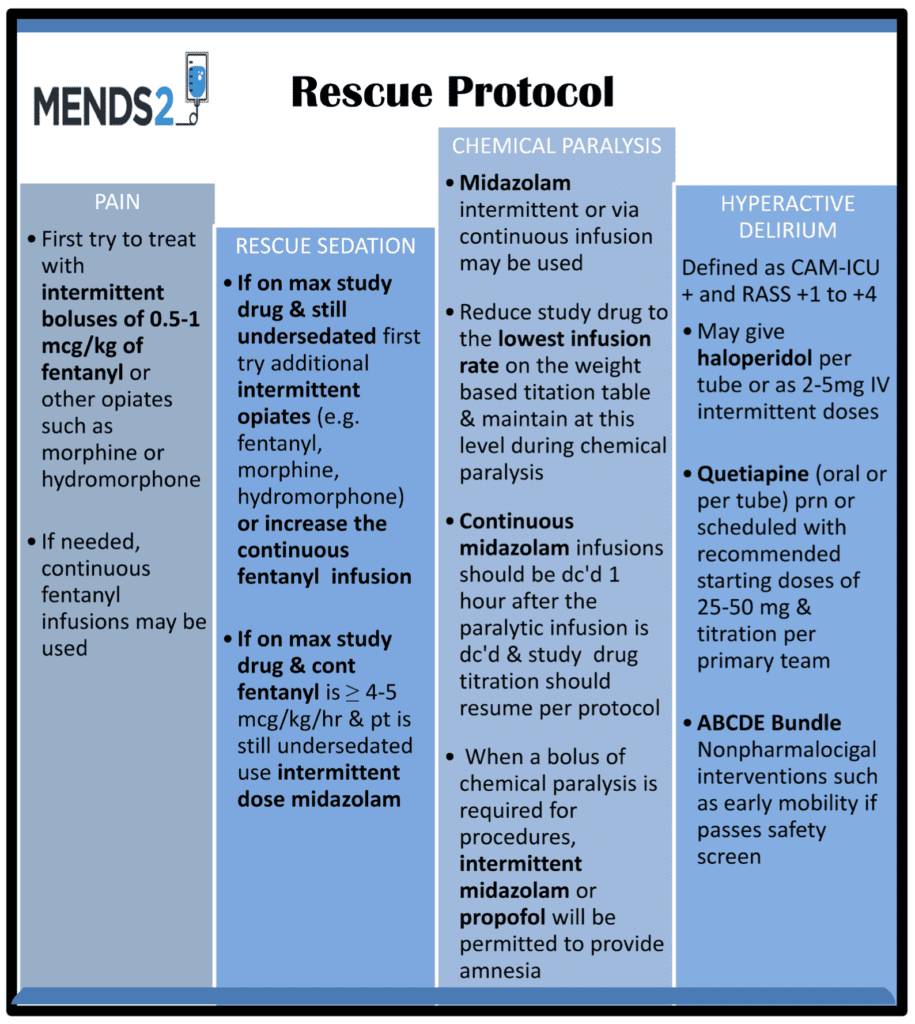
- Rescue Protocol:
Outcomes:
- Primary: Median Days alive without delirium or coma during 14d intervention period
- Secondary:
- Ventilator-free days at 28d
- Death at 90d
- Age-adjusted total score on Telephone Interview for Cognitive Status questionnaire (TICS-T) at 6 months
- TICS-T scores range from 0 to 100 with 50 +/-1- and lower scores indicating worse cognition
Inclusion:
- Sequentially admitted adults to the ICU
- Suspected or known infection
- Treated with continuous sedation for invasive mechanical ventilation
Exclusion:
- Baseline severe cognitive impairment
- Pregnant or breast feeding
- Blind, deaf, or unable to understand approved languages
- Second- or third-degree heart block
- Persistent bradycardia requiring intervention
- Allergy to dexmedetomidine or propofol
- Indication for benzodiazepines
- Anticipated to have immediate discontinuation of mechanical ventilation
- Expected to have neuromuscular blockade for more than 48hrs
- Moribund state
- Had received mechanical ventilation for >96hrs before meeting all inclusion criteria
Results:
- 432 patients randomized
- 422 assigned to receive trial drug and included in the analysis
- Dexmedetomidine median dose = 0.27 ug/kg/hr
- Propofol median dose = 10.21ug/kg/min
- Overall time spent at target sedation was ≈60% in both groups
- Median Fentanyl Dose on Days Administered:
- Dexmedetomidine: 68 ug/hr
- Propofol: 56ug/hr
- Median Number of Days Alive Without Delirium or Coma
- Dexmedetomidine: 10.7d
- Propofol: 10.8d
- OR: 0.96; 95% CI 0.74 to 1.26; p = 0.79
- Median Ventilator Free Days at 28d
- Dexmedetomidine: 23.7d
- Propofol: 24.0d
- OR 0.98; 95% CI 0.63 to 1.51
- 90d Mortality:
- Dexmedetomidine: 38%
- Propofol: 39%
- HR 1.06; 95% CI 0.74 to 1.52
- Median TICS-T Score at 6 Months:
- Dexmedetomidine: 40.9
- Propofol: 41.4
- OR 0.94; 95% CI 0.666 to 1.33
- Safety End points were similar in the two groups
Strengths:
- Asks a clinically important question
- Multicenter, double-blind, randomized controlled trial
- Pfizer supplied dexmedetomidine but had no role in the design or conduct of the trial, analysis of the data, or writing the manuscript
- Before group assignments were unmasked, the statistical analysis plan was published
- Researchers, clinicians (except bedside nurses), patients, and families were blinded to group assignments
- Study drugs were prepared in identical intravenous fluid bags covered with opaque plastic bags to be administered in units of milliliters per hour to maintain study masking
- All patients had daily awakening, breathing coordination, choice of sedation, delirium monitoring/management, and early mobility bundles followed
- Used well validated tools (i.e. RASS, CAM-ICU, and Critical Care Pain Observation Tool) for assessments of sedation, delirium, and pain
- Proactively assessed for unblinding
- Assessed >90% of patients eligible at 6 months after randomization
Limitations:
- Unblinding in 14% of patients with similar frequency in the two groups and 10% crossover rate which could affect outcomes
- Low recruitment, which required adjustment of sample size
- Despite evaluating 4840 patients, the majority met exclusion criteria and 537 declined to participate and 471 were not included due to medical team declining participation
Discussion:
- The goal of this study was to examine the effects of propofol and dexmedetomidine titrated for light sedation in mechanically ventilated patients. Although the researchers used low doses of each medication more than half of each of these groups were moderately deeply sedated or comatose/unarousable:
- Doses of dexmedetomidine and propofol that were used were TINY (median dose 0.27ug/kg/hr and 10ug/kg/min)
- 38% of patients in the low dose dexmedetomidine group were either RASS -4 (Deep Sedation) or -5 (unarousable) and additional 14% were moderate sedation RASS -3. So, a total of 52% of these patients were moderate sedation to comatose in low dose dexmedetomidine group.
- 36% of patients in the low dose propofol group were either RASS -4 (Deep Sedation) or -5 (unarousable) and additional 16% were RASS -3 (moderate sedation). So, a total of 54% of these patients were moderate sedation to comatose in low dose dexmedetomidine group.
- Patients did get lots of fentanyl (median dose 60ug/hr or =1440mcg/day = 144mg/day of morphine)
- Looking at the rescue protocol you can see that fentanyl and other sedatives were used a lot for rescue sedation
- Additional sedation needed in both groups
- In the dexmedetomidine group
- 53% needed rescue midazolam
- 42% needed rescue antipsychotic
- 13% needed open label propofol use
- In the propofol group
- 43% needed rescue midazolam
- 42% needed rescue antipsychotic
- 8% needed open label propofol use
- In the dexmedetomidine group
- Due to a lower than anticipated recruitment a protocol amendment was made in March 2017 to lower the enrollment target from 530 to 420 patients to provide 85% power to detect a 1.5d difference in days alive without delirium or coma between groups and 80% power to detect a 12% absolute difference in 90d mortality, assuming an expected mortality of 30% in the propofol group, which was seen in this trial
- All data analyzed in a modified intention to treat analysis which was prespecified as all patients who underwent randomization and received a trial drug
- Despite using light sedation 25% of patients had cognitive dysfunction despite light sedation approaches after their critical illness
- In the end, RASS goals representing moderate to comatose states were achieved in more than half of these patients. Although I commend the authors for trying to conduct this trial, I really don’t think it was a head-to-head trial of low doses of dexmedetomidine vs propofol as too many other medications were used to heavily sedate these patients. I believe it’s unreasonable to expect this low of a dose of either medication could truly achieve the intended goals. I would be cautious to conclude much based on this study, and would not switch my practice. If anything, it shows that it is hard to achieve light sedation (RASS -2) with low doses of either medication, and perhaps higher doses of each are needed which may come with side effects or other medications like opiates (analgesia) and antipsychotics, limited benzodiazepines, and other medications are often needed.
- Should try to use multimodal approach:
- Pain = Analgesics
- Anxiety = Sedatives
- Delirium = Antipsychotics
Author Conclusion: “Among mechanically ventilated adults with sepsis who were being treated with recommended light-sedation approaches, outcomes in patients who received dexmedetomidine did not differ from outcomes in those who received propofol.”
Clinical Take Home Point: Among critically ill patients with sepsis who were receiving mechanical ventilation, it is not surprising that dexmedetomidine did not lead to better outcomes than propofol in any outcome. To me, it is unreasonable to expect low doses of dexmedetomidine and propofol (median dose 0.27ug/kg/hr and 10ug/kg/min) could achieve the intended RASS goals for light sedation. In fact, a lot of fentanyl and other rescue medications were used to keep patients sedated.
References:
- Hughes CG et al. Dexmedetomidine or Propofol for Sedation in Mechanically Ventilated Adults with Sepsis. NEJM 2021. PMID: 33528922[Access on Read by QxMD]
- Devlin JW et al. Clinical Practice Guidelines for the Prevention and Management of Pain, Agitation/Sedation, Delirium, Immobility, and Sleep Disruption in Adult Patients in the ICU. Crit Care Med 2018. PMID: 30113379
For More Thoughts on This Topic Checkout:
- PulmCrit: MENDS2 – Fentanyl or Fentanyl for Sedation in Mechanically Ventilated Adults with Sepsis
- The Bottom Line: MENDS2
Post Peer Reviewed By: Frank Lodeserto, MD (Twitter: @FrankLodeserto)
The post The MENDS2 Trial: Dexmedetomidine vs Propofol for Sedation appeared first on REBEL EM - Emergency Medicine Blog.
