
 Background: Many Out-of-Hospital Cardiac Arrest (OHCA) are attributable to ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT). Both are said to be treatable presentations of OHCA, due to their responsiveness to defibrillation. VF and VT can persist or recur after defibrillation with an inverse relationship between the duration of OHCA, the recurrences of arrhythmias, and ultimately resuscitation outcomes.
Background: Many Out-of-Hospital Cardiac Arrest (OHCA) are attributable to ventricular fibrillation (VF) or pulseless ventricular tachycardia (VT). Both are said to be treatable presentations of OHCA, due to their responsiveness to defibrillation. VF and VT can persist or recur after defibrillation with an inverse relationship between the duration of OHCA, the recurrences of arrhythmias, and ultimately resuscitation outcomes.
Amiodarone and lidocaine are both recommended by the advanced cardiovascular life support (ACLS) guidelines to help promote successful defibrillation in refractory ventricular fibrillation or pulseless ventricular tachycardia and to prevent recurrences. In previous randomized controlled trials patients receiving amiodarone vs placebo or lidocaine in OHCA were more likely to have return of spontaneous circulation (ROSC) and to survive to hospital admission. However the effects of amiodarone on survival to hospital discharge or neurologic outcome still remain uncertain. Should we be using anti-dysrhythmic drugs in out-of-hospital cardiac arrest?
What Trial Are we Discussing?
Kudenchuk PJ et al. Amiodarone, Lidocaine, or Placebo in Out-of-Hospital Cardiac Arrest. NEJM 2016. [Epub Ahead of Print]
What They Did:
- Randomized, double-blind trial from 10 North American Sites
- Parenteral amiodarone, lidocaine, or saline placebo along with standard care in adults with non-traumatic OHCA
- Amiodarone 300 mg IV (150 mg if < 45.4 kg)
- Lidocaine 120mg IV (60 mg if < 45.5 kg)
- 0.9% Normal Saline IV
- Inclusion:
- Shock-refractory ventricular fibrillation or pulseless ventricular tachycardia
- At least one shock
- Vascular access
- Exclusion:
- Patient already receiving open-label IV lidocaine or amiodarone during resuscitation
- Known hypersensitivity to amiodarone or lidocaine
- Known advance directive
- Protected populations (child, pregnant, prisoner)
Outcomes:
- Primary: Survival to hospital discharge
- Secondary: Favorable neurologic function at discharge (modified Rankin Score < 3)
Results:
-
3026 patients included
- 974 Amiodarone arm
- 993 Lidocaine arm
- 1059 Placebo arm
Strengths:
- Double Blinded Randomized Clinical Trial
- Baseline characteristics between groups were evenly matched
- 99.5% Patient Follow up
- No differences in pre-shock pauses, compression rate, compression depth, or CPR fraction between groups during first 10 minutes after pad placement
Limitations:
- Post arrest care was not standardized between hospitals and this could create imbalances between trial groups
- Trial was powered to detect a 6% absolute differences in survival to hospital discharge. It is possible that there is a smaller difference that is a true difference but this study cannot determine this.
- Selection bias could have influenced trial enrollment
- Enrollment of patients whose condition at randomization who had little to no chance of survival may have diluted the results of this study
Discussion:
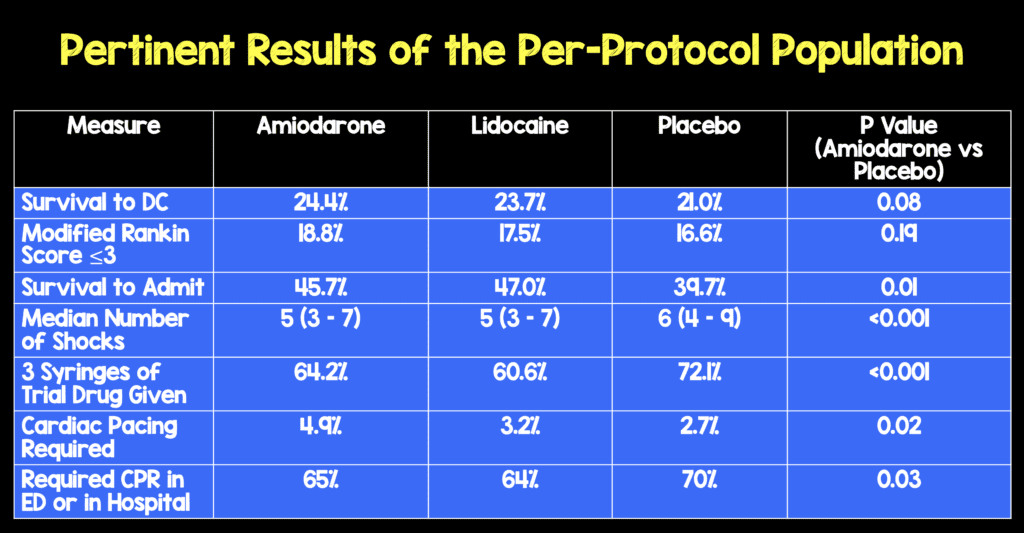
- In a subgroup analysis, Amiodarone and Lidocaine had a higher rate of survival to hospital discharge than placebo among patients with witnessed OHCA
- Amiodarone 27.7% (171 pts)
- Lidocaine 27.8% (176 pts)
- Placebo 22.7% (155 pts)
- Amiodarone vs Placebo 5% (95% CI 0.3 – 9.7; p = 0.04)
- Lidocaine vs Placebo 5.2% (95% CI 0.5 – 9.9; p = 0.03)
- Interestingly amiodarone and lidocaine facilitated termination of ongoing or recurrent ventricular fibrillation or pulseless ventricular tachycardia with fewer shocks than placebo, higher rates of hospital admission, and lesser need for CPR or antiarrhythmic therapies during hospitalization
- The time to treatment was 19 minutes on average which could attenuate the effectiveness of antiarrhythmic interventions (i.e. cellular injury and physiological derangements may be irreversible)
Author Conclusion: “Overall, neither amiodarone nor lidocaine resulted in a significantly higher rate of survival or favorable neurologic outcome than the rate with placebo among patients with out-of-hospital cardiac arrest due to initial shock-refractory ventricular fibrillation or pulseless ventricular tachycardia.”
Clinical Take Home Point: Both amiodarone and lidocaine showed similar benefits with respect to short-term outcomes in OHCA due to initial shock refractory ventricular fibrillation or pulseless ventricular tachycardia compared to placebo, but this did not result in a higher rate of survival to hospital discharge or favorable neurologic outcomes.
References:
- Kudenchuk PJ et al. Amiodarone, Lidocaine, or Placeboe in Out-of-Hospital Cardiac Arrest. NEJM 2016. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- Ryan Radecki at EMLit of Note: Amiodarone, Lidocaine, or…Nothing
- Celia Bradford at The Bottom Line: Amiodarone, Lidocaine or Placebo in Out-of Hospital Cardiac Arrest
- Thomas D at ScanCrit: CPR and Amiodarone
- Rory Spiegel at EM Nerd: The Case of the Perfect Imperfection
- Natalie May at St. Emlyn’s: Arrested Developments
- Mike Winters & Amal Mattu at Resuscitation: Amio vs Lidocaine vs Placebo in Cardiac Arrest
- Anton Helman at EM Cases: Journal Jam 7 – Amiodarone vs Lidocaine vs Placebo in Cardiac Arrest: The ALPS Trial
- Ken Milne at The SGEM: SGEM#162: Not Stayin’ Alive More Often with Amiodarone or Lidocaine in OHCA
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)
The post ALPS: Amiodarone, Lidocaine or Placebo Study in OHCA appeared first on REBEL EM - Emergency Medicine Blog.
