
 Background: Anyone who has had a corneal abrasion knows how painful it can be. Unfortunately, traditional analgesic agents (ibuprofen, acetaminophen, opiates etc) are ineffective in relieving pain. Topical anesthetic drops can be diagnostic of superficial eye pathology and are routinely used prior to slit-lamp examination. They provide immediate relief in the emergency department and have been shown to be effective and safe (Limited evidence has not shown significant adverse events) as short-term outpatient therapy (REBEL EM). The biggest concern with the use of topical anesthetic agents as outpatient therapy is delayed healing, which is what most evidence has focused on. This may occur with long-term use, but not been seen in studies of short-term use of topical anesthetics (REBEL EM). Use of short-term topical anesthetics further has the potential to reduce the use of opioids for analgesia for this indication. Recent published literature on topical anesthetic for this indication have focused on harms. There is scant literature detailing their benefit.
Background: Anyone who has had a corneal abrasion knows how painful it can be. Unfortunately, traditional analgesic agents (ibuprofen, acetaminophen, opiates etc) are ineffective in relieving pain. Topical anesthetic drops can be diagnostic of superficial eye pathology and are routinely used prior to slit-lamp examination. They provide immediate relief in the emergency department and have been shown to be effective and safe (Limited evidence has not shown significant adverse events) as short-term outpatient therapy (REBEL EM). The biggest concern with the use of topical anesthetic agents as outpatient therapy is delayed healing, which is what most evidence has focused on. This may occur with long-term use, but not been seen in studies of short-term use of topical anesthetics (REBEL EM). Use of short-term topical anesthetics further has the potential to reduce the use of opioids for analgesia for this indication. Recent published literature on topical anesthetic for this indication have focused on harms. There is scant literature detailing their benefit.
Paper: Shipman S et al. Short-Term Topical Tetracaine is Highly Efficacious for the Treatment of Pain Caused by Corneal Abrasions: A Double-Blind, Randomized Clinical Trial. Ann Emerg Med 2020. PMID: 33121832 [Open in Read by QxMD]
Clinical Question: How effective is 24-hour home use of topical tetracaine in patients with uncomplicated corneal abrasions compared to placebo?
What They Did:
- Single-center, prospective, double-blind, placebo-controlled, randomized trial of adults with uncomplicated corneal abrasions
- Approached 118 patients from Jan 2015 to Sept 2017
- All patients received:
- Polymyxin B sulfate/trimethoprim sulfate with instructions to instill 2 drops q4hrs into the affected eye
- Hydrocodone/Acetaminophen 7.5/325mg (12 tablets) and instructed to use 1 or 2 tablets PRN q6hrs for breakthrough pain
- Patients randomized 1:1 to:
- Topical tetracaine 0.5% 1 drop q30min PRN x24hrs
- Topical placebo 1 drop q30min PRN x24hrs (artificial tear solution)
Outcomes:
- Primary: Overall numeric rating scale pain score measured at 24 to 48 hours of ED follow-up (From 0 to 10cm)
-
Secondary:
- Amount of hydrocodone ingested for breakthrough pain
- Any adverse events
Inclusion:
- Age 18 to 80 years
- Presented to the ED with suspected acute corneal abrasion
- Gave written informed consent
Exclusion:
- Wore contact lenses
- Previous corneal surgery or transplant in affected eye
- Presented >36hrs after injury
- Grossly contaminated foreign body
- Coexisting ocular infection
- Pregnancy
- Retained foreign body
- Penetrating eye injury
- Immunosuppression
- Allergy to study medication
- Inability to attend follow-up
- Inability to fluently read and speak English or Spanish
- Any injury requiring urgent ophthalmologic evaluation (i.e. large or complicated abrasions with vision loss, corneal ulcers, or corneal lacerations)
Results:
- 111 adults in final analysis
- Baseline pain rating was 7 in both groups
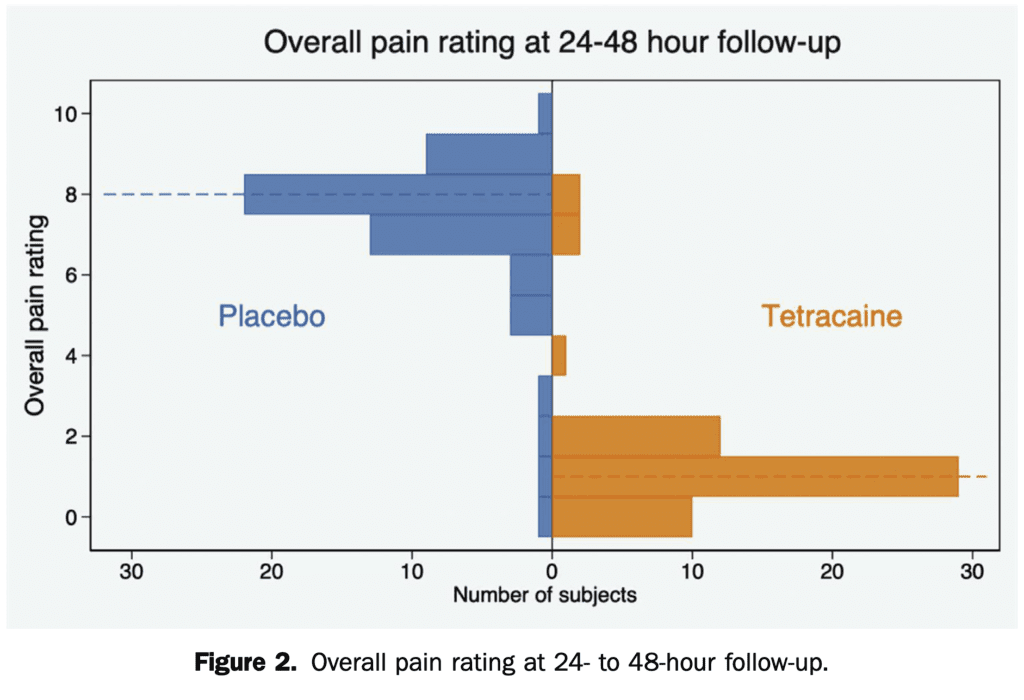
- Overall Numeric Rating Scale Pain Score at 24 to 48 Hours:
- Tetracaine: 1 out of 10
- Placebo: 8 out of 10
- Absolute Difference: 7; 95% CI 6 to 8; p <0.001
- Median Hydrocodone Tablet Use:
- Tetracaine: 1 Tablet
- Placebo: 7 Tablets
- Complication Rate:
- Tetracaine: 3.6%
- Placebo: 11%
- Small Residual Corneal Abrasion on Repeat ED Slit-Lamp Examination:
- Tetracaine 10/56 (18%)
- Placebo 6/56 (11%)
- 95% CI -6.4 to 20.4
- Not statistically significantly different
Strengths:
- Prospective, randomized, double-blind, placebo-controlled trial
- Patients enrollment could occur at any time 7 days a week
- Collected data on all patients who declined study participation or met any exclusion criteria
- Enrolling physicians were blinded to the randomization plan
- All patients in both groups received their allocated intervention
- Baseline pain score was similar between 2 groups
- Data for initial ED follow up of primary and secondary endpoints was collected in 95% of tetracaine group and 93% of the placebo group
Limitations:
- Packaging of solutions was not identical which could lead to unblinding
- Placebo: 4 separate 0.5mL ampules of artificial tear solution
- Tetracaine: A single 2mL bottle
- Burning nature of tetracaine may have unintentionally unblinded patients which could have biased outcomes
- 90 out of 111 patients did not follow up at ophthalmology at 1 week. It is possible that complications were missed or developed later (The numbers were equal in both groups)
- Study too small and not powered for rare adverse events
- Excluded patients with large or complicated corneal abrasions, penetrating eye injuries, contact lens wearers, patients with previous corneal surgery in the affected eye, greater than 36 hours after injury, grossly contaminated foreign bodies, and coexisting ocular infections. Therefore, results of this trial cannot be extrapolated to these groups
Discussion:
- Power calculations showed that 60 patients per group would have 95% power to detect a minimum clinical difference in pain scores of 1.5cm on a 10-cm NRS. The study was underpowered for this outcome detection
- In the study, additional study drops were collected and disposed of to ensure no patients used them for a longer period and all patients were asked to follow up with a study ophthalmologist within 1 week of their initial visit. This may not be a reality in many EDs and for patients. This is evidenced by the fact that 90 out of 111 patients did not return for their 1-week ophthalmology follow-up
- For more on topical NSAIDs checkout Jacob Avila’s post on REBEL EM [Link is HERE]
- For more on how to dilute, or limit the volume of topical anesthetics, checkout my post on REBEL EM [Link is HERE]
- Ensuring appropriate follow up is key in these patients. This can be done by calling an ophthalmologist to schedule the appointment or simply having the patient come back to the ED for a 24 to 48 hour follow up appointment. I personally, always get the phone number of these patients and call them to ensure they are not using the drops past 48 hours and that they are doing better.
Author Conclusion: “Short-term topical tetracaine is an efficacious analgesic for acute corneal abrasions, is associated with less hydrocodone use compared with placebo, and was found to be safe in this sample.”
Clinical Take Home Point: 24 hours of topical 0.5% tetracaine for uncomplicated corneal abrasions reduced pain scores at 24 to 48 hours and was opioid sparing for breakthrough pain compared to placebo. Larger trials would be needed to rule out rare adverse events but adding this study to previous data it seems that 24 to 48 hours of topical anesthetic use for uncomplicated corneal abrasions is safe. It is important to emphasize the need for follow-up, short-term use, and return precautions in these patients to avoid long-term use of these medications.
References:
- Shipman S et al. Short-Term Topical Tetracaine is Highly Efficacious for the Treatment of Pain Caused by Corneal Abrasions: A Double-Blind, Randomized Clinical Trial. Ann Emerg Med 2020. PMID: 33121832 [Open in Read by QxMD]
- Swaminathan, A et al. The Safety of Topical Anesthetics in the Treatment of Corneal Abrasions: A Review. J Emerg Med 2015. PMID: 26281814 [Open in Read by QxMD]
For More Thoughts on This Topic Checkout:
- REBEL EM: Topical Anesthetic Use on Corneal Abrasions
- REBEL EM: Topical Pain Control for Corneal Abrasions
- The Bottom Line: SHIPMAN
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post Corneal Abrasions and Short-Term Topical Tetracaine appeared first on REBEL EM - Emergency Medicine Blog.


