
 Background: Despite access to vaccines in developed countries, COVID19 remains a nimble and persistent foe. While case counts wax and wane the virus continues to spread and mutate. The rapid development of vaccines has been helpful but the distribution across the world has remained scattered.
Background: Despite access to vaccines in developed countries, COVID19 remains a nimble and persistent foe. While case counts wax and wane the virus continues to spread and mutate. The rapid development of vaccines has been helpful but the distribution across the world has remained scattered.
The main focus of therapies against the SARS-CoV2 virus/COVID-19 has been:
Anti-virials: These agents have been focused mostly on decreasing viral replication (ex. Remdesivir).
Steroids: Steroids, as we all know are cheap and widely available but they broadly downregulate many immune responses and also carry various side effects such as immunosuppression, hyperglycemia and delirium.
Monoclonal Antibodies: These agents are more narrowly focused and work by blocking a single cytokine pathway or other immune pathway. Examples have been tocilizumab which is an IL-6 inhibitor. There are other types of monoclonal antibodies specific to the SARSCoV2 virus as well. One of these specific monoclonal antibodies is bamlanivimab which binds to the receptor binding domain on the spike protein preventing its attachment. Monoclonal antibodies are narrowly focused which may decrease side effects compared to other agents like steroids. However, these medications can be expensive and because of their narrow focus, may not have enough pro-inflammatory downregulation to really add benefit.
Janus Kinase Inhibitors (JAK Inhibitors): These agents now being explored as a useful class of medications that can downregulate the hyperinflammatory response seen by SARSCoV2/COVID-19. JAK Inhibitors can downregulate multiple cytokine pathways therefore giving it a broader spectrum than monoclonal antibodies, but not so broad as steroids. For example, Jak 1 and 2 Inhibitors have been shown to downregulate IL-2, IL-6, IL-12, as well as interferons. Baricitinib is a JAK1 and JAK 2 inhibitor which has shown safety and effectiveness already in hospitalized patients with COVID-19.
The original COV-BARRIER study for Baricitinib plus standard of care (SOC) for hospitalized patients assessed the impact of Baricitinib on the escalation of oxygen requirements from high flow/positive pressure to mechanical support. What it did not address was the impact of Baricitinib in patients who are already on a ventilator or ECMO. This study, funded by Eli Lilly, sought to fill that void and give intensivists the data to shape their practice with COVID ICU patients.
Paper: Marconi V et al. Baricitinib plus Standard of Care for Hospitalized Adults with COVID-19 on Invasive Mechanical Ventilation or Extracorporeal Membrane Oxygenation: Results of a Randomized, Placebo-Controlled Trial. Lancet Respir Med 2021. PMID: 34480861
Clinical Question: What is the safety and efficacy of Baricitinib in patients who are on mechanical ventilation or on ECMO when given with the standard of care (which includes steroids)?
What They Did:
- This addendum trial from COV-BARRIER was an international (4 countries) double-blinded, placebo controlled, phase 3 trial across 18 centers. Participants were randomized 1:1 to receive enteral baricitinib 4mg PO qD or placebo for 14 days or until discharge. Dosing was reduced for lower GFR (eGFR ≥30 to <60 mL/min/1.73 m2 received 2mg PO qD). They were followed up at 28 days after their last dose of the medication.
Population:
- Inclusion Criteria:
- >18yo, hospitalized with lab confirmed COVID-19 and already mechanically ventilated or on ECMO
- Labs with at least one elevated inflammatory marker (i.e. Ferritin, C-reactive protein, D-dimer, or lactate dehydrogenase)
- Exclusion Criteria:
- High dose steroids (ex. dexamethasone doses >20mg/day or equivalent dose of prednisone) for at least 14 days within a month of the study start date unless needed for another underlying comorbidity.
- Patients on immunosuppressants, biologics, T cell or B cell targeted therapies, interferon, or JAK inhibitors; received convalescent plasma or intravenous immunoglobulin for COVID-19; or suspected serious active bacterial, fungal, or other infection, or untreated TB.
Outcomes:
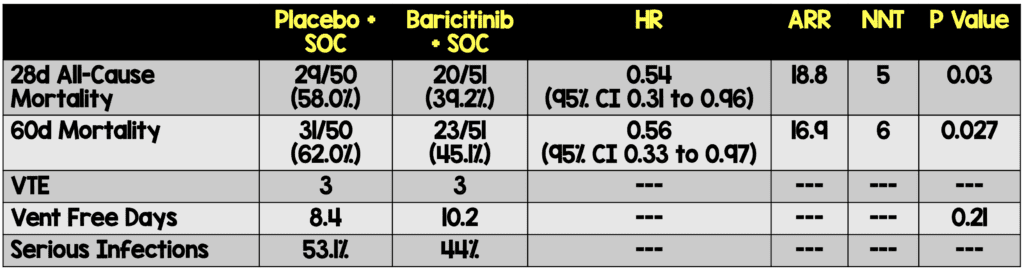
- Mortality day 28 and 60
- Ventilator free days
- Overall improvement in clinical status
- Hospital length of stay
- Time to recovery through day 28
Results:
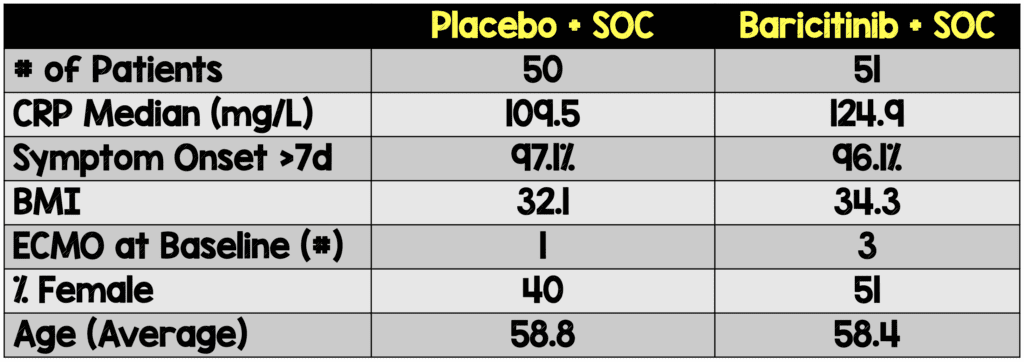
- 86.1% of participants were receiving systemic corticosteroids and only 2.0% were receiving remdesivir
Critical Results:
Strengths:
- 1st phase 3 study to evaluate baricitinib in addition to standard of care including antivirals, anticoagulants, and corticosteroids in patients receiving invasive mechanical ventilation or ECMO
- Baseline characteristics and demographics were balanced between treatment groups
- The study is double blinded, placebo controlled, and performed across multiple countries.
- The efficacy data was analyzed with an intent-to-treat.
- This study provides insight into baricitinib vs placebo with the use of steroids
- This study provides new insight into the safety of baricitinib inpatients who are already mechanically ventilated.
- The study provides mortality data for day 60.
Limitations:
- Small sample size with ~ 50 patients per group
- Only 4 patients on ECMO at baseline of the 114 participants.
- Only 2 of the participants were on remdesivir at baseline so the SOC for this paper is truly only concurrent corticosteroid treatment.
- There is no data regarding the impact on inflammatory markers during or after treatment
Discussion:
- This is a well-designed placebo controlled international double blinded study that is relevant for the ICU by providing data on ventilated/ECMO patients
- The overall findings are that at 28 days there is a mortality benefit when baricitinib is added to the standard of care for these critically ill patients
- The majority of participants were >7 days into symptoms so the fact that a benefit was still seen makes this a clinically relevant medical intervention.
- This study highlights patients who received baricitinib did not suffer an increase in serious or opportunistic infections as was often rumored during early use after emergency use authorization.
- There was no statistically significant difference in ventilator free days
Author’s Conclusion:
“In critically ill patients with COVID-19 already receiving IMV/ECMO, treatment with barcitinib as compared to placebo (in combination with SOC, including corticosteroids) showed mortality HR of 0.56, corresponding to a 44% relative reduction at 60 days. This is consistent with the mortality reduction observed in less severely ill hospitalized primary COV-BARRIER study population.”
Our conclusion:
When studied in a small sample size Baricitinib demonstrated a modest mortality benefit in mechanically ventilated COVID patients.
Clinical Bottom Line:
In patients admitted to the hospital with COVID-19, who have elevated systemic inflammatory markers, and rapidly increasing oxygen requirements, one thing to consider is the addition of baricitinib to corticosteroids. These results still need to be reproduced in a larger randomized control trial, but this recommendation is supported by the National Institutes of Health COVID-19 recommendations.
Guest Post By:

Post Peer Reviewed By: Frank Lodeserto, MD and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post COV-BARRIER Addendum Study: Baricitinib in ECMO/Mechanically Ventilated Patients with COVID-19 appeared first on REBEL EM - Emergency Medicine Blog.
