Background: Throughout the COVID-19 pandemic, numerous therapeutic agents have been repurposed and applied empirically and within clinical trials. Prophylactic medications for COVID-19 could have a huge benefit, but studies to date haven’t panned out. Initially many therapeutic medications were used late in illness, and one of the criticisms of these negative studies was that the drugs were applied too late in the disease and therefore didn’t have any potential for benefit. There were also numerous studies showing associations of benefit, but subsequent randomized clinical trials have failed to prove effectiveness in reducing mortality (i.e. Remdesivir, hydroxychloroquine, lopinavir/ritonavir, colchicine, convalescent plasma, monoclonal antibody therapy).
Ivermectin is an anti-parasitic medication that has been the focus of speculation as an anti-viral, and anti-inflammatory medication against SARS-CoV-2 and COVID-19. In this post we will review some of the current evidence in using Ivermectin as a prophylactic and therapeutic agent in COVID-19.
Clinical Question: Does Ivermectin demonstrate efficacy in prophylaxis and treatment of COVID-19?
In Vitro Evidence [2]
- Cells infected with SARS-CoV-2 RNA
- Added Ivermectin or nothing (control) to cells and analyzed RT-PCR for replication of SARS-CoV-2 RNA at days 0 to 3
- 24h = 93% reduction in viral RNA present
- 48h = 99.8% reduction in viral RNA present
- By 48hrs there was an ≈5000-fold reduction in viral RNA in Ivermectin treated cells compared to control samples
- With a single dose of Ivermectin viral replication was controlled effectively eliminating all viral material by 48hrs
- No toxicity observed at anytime
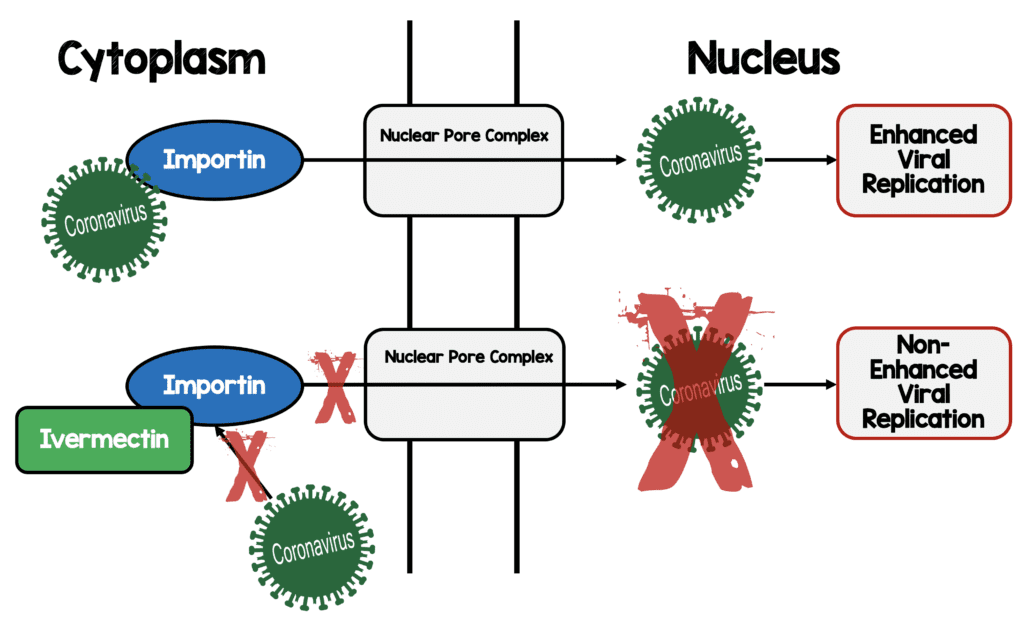
- Proposed anti-viral mechanism of action
Studies You’ve Heard About But Determined to be Fraudulent
- Largest and best RCT [3] on the use of Ivermectin (600 patients) from Egypt BUT…
- Study retracted for ethical concerns of plagiarism and falsified data (Link is HERE)
- Study out of Lebanon [11] in 100 asymptomatic patients retracted due to blocks of details of 11 patient’s data being copied and pasted repeatedly (Link is HERE)
- MATH+ therapy touted by Pierre Kory also retracted due to flawed results (Link is HERE)
Observational Data from Countries Using Prophylactic Ivermectin vs Those not Using Prophylactic Ivermectin [4]
- Grouped countries into three different categories:
- Include ivermectin in prophylaxis
- Do not include ivermectin in prophylaxis
- Do not do prophylaxis
- Compared COVID-19 incidence between these three groups:
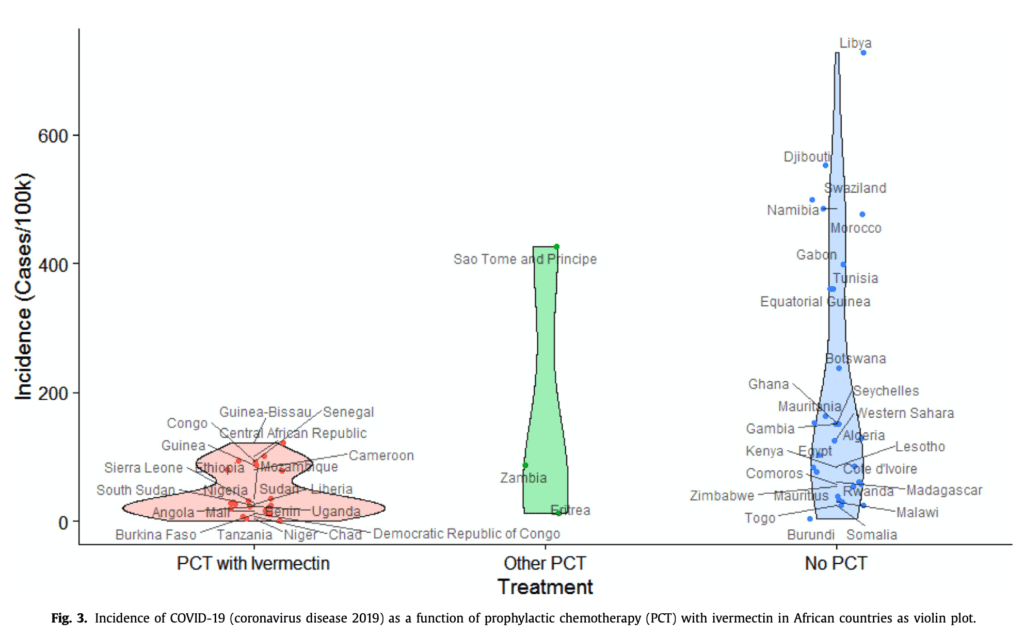
- The fact that prophylaxis without ivermectin also showed a strong and statistically significant association with COVID-19 incidence suggests that other drugs could include additional candidates for the treatment and/or prevention of COVID-19
- Conclusion:There seems to be an association rather than a causation of ivermectin use reducing COVID-19 incidence. However, we don’t know the rates of COVID-19 in these countries, crowding, testing availability, etc. There’s simply too much unknown information to make anything of this paper other than a hypothesis to study.
Evidence from Iraq [5]
- Randomized controlled trial of 140 patients:
- Ivermectin-Doxycycline reduced mean time to recovery from 17.9 to 10.61d in all recruited patients
- In mild to moderate patients this reduction was from 13.66d to 6.34d
- In severe patients this reduction was from 24d to 20d
- This can have a tremendous effect on lowering the burden of disease and quickly freeing up hospital beds to other patients
-
Limitations:
- Convenience sample: We have no idea how many patients total could have gotten treatment
- Unclear what earlier means as there were no critical patients in the SOC arm
- Non-blinded: Everyone knew what they were taking
- Randomization method is flawed
- Small study
- No clear definition of recovery making this a subjective outcome that can bias the study
- No idea if it’s the ivermectin or the doxycycline making the difference if you believe the difference
- No information on patients to see if groups were balanced (i.e. demographics, underlying disease, etc.)
- Conclusion:On the surface it seems Ivermectin with doxycycline reduced the time to recovery, percentage of patients who progress to more advanced stages of disease and reduced mortality. However, there were so many methodological issues I would not put any weight in these conclusions.
The ICON Study [7]
- Retrospective observational cohort trial of consecutive patients hospitalized with COVID-19 from 4 hospitals in Florida
- Reviewed charts of patients with COVID-19 treated with and without Ivermectin
- This is the study that got everyone’s attention on Ivermectin as it was published in CHEST
- Primary:All-cause in-hospital mortality
-
Results:
- 280 patients (173 treated with Ivermectin and 107 without)
- Patients received at least one dose of Ivermectin 200mcg/kg + standard care vs standard care alone
- A second dose of 200mcg/kg of Ivermectin could be given on day 7 at the discretion of the treating clinician
- Univariate analysis showed lower mortality in ivermectin group (15.0%vs 25.2%; OR 0.52; 95% CI 0.29 to 0.96; p = 0.03)
- Mortality also lower in patients with severe pulmonary involvement (need for FiO2 ≥50%, NIV, or IMV)
- 8% vs 80.7%; OR 0.15; 95% CI 0.05 to 0.47; p = 0.001
- No difference in extubation rates or length of state
- After multivariate adjustment for confounders, mortality difference remained significant (OR 0.27; 95% CI 0.09 to 0.80; p = 0.03)
- Propensity matching was also used and found mortality to be significantly lower in ivermectin group (13.3% vs 24.5%; OR 0.47; 95% CI 0.22 to 0.99; p < 0.05) an 11.2% absolute risk reduction with a NNT of 8.9
-
Limitations:
-
Biggest limitation of this studyis that patients in the Ivermectin group got steroids far more commonly than those who didn’t:
- Unmatched Cohort: 39.8% vs 19.6%
- Matched Cohort: 25.5% vs 21.4%
- More of the control group was enrolled in the 1stweeks of the study suggesting a timing bias. We get better at caring for a new disease as time goes on
-
Biggest limitation of this studyis that patients in the Ivermectin group got steroids far more commonly than those who didn’t:
- Conclusion:According to the authors, Ivermectin treatment was associated with lower mortality during treatment of COVID-19, especially in patients with severe pulmonary involvement. However more patients in the Ivermectin arm received corticosteroids than those who didn’t and the benefits seen in this trial may simply be due to this fact.
Summary of Clinical Evidence for Ivermectin Against COVID-19 [1]
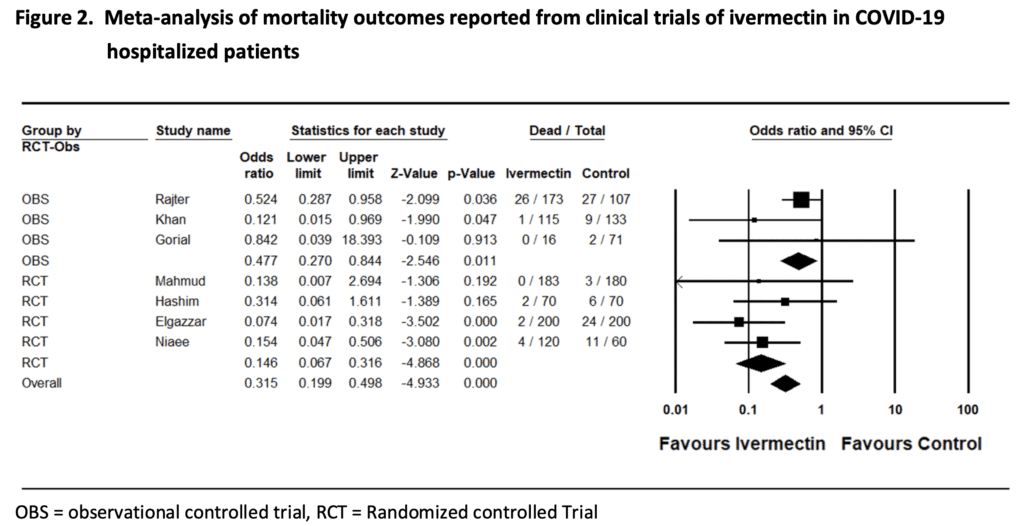
Again, on the surface this looks promising, however going through the individual trials is essential, as the conclusions of any meta-analysis are only as good as the individual trials that go into it. Many of these individual trials have methodological flaws that limit the utility of this analysis.
Poorly Done Trial + Poorly Done Trial + Poorly Done Trial = Poor Conclusion
Evidence from Spain [8]
What They Did:
- Pilot, randomized, double-blind, single-center, parallel-arm, superiority placebo-controlled trial performed in Spain
- Evaluating single dose of ivermectin to reduce transmission of SARS-CoV-2 when administered early after disease onset
- Consecutive patients with non-severe COVID-19 and no risk factors for complicated disease presenting to the ED
- All enrollments occurred within 72hrs of onset of fever or cough
- Patients randomized 1:1 to receive:
- Ivermectin 400mcg/kg single dose
- Placebo single dose
Outcomes:
- Primary: Proportion of patients with detectable SARS-CoV-2 RNA by PCR from nasopharyngeal swab at day 7 post treatment
Results:
- 24 total patients
- 100% had symptoms at recruitment
- Ivermectin group had significantly lower viral loads at day 4 (3-fold lower) and day 7 (18-fold lower)
- Confidence intervals crossed at all time points making this not statistically significant
- Symptoms
- Fewer days of any symptoms, however driven by anosmia/hyposmia (50% less) and cough (30% less)
- No patients from either group progressed to severe disease
- IgG Titers:
- All patients in both groups seroconverted by day 21 post treatment
- Lower IgG titers at day 21 post treatment in the ivermectin group (4.7 vs 7.5)
- Not statistically significant
- Safety:
- No severe adverse events in either group
- More dizziness and blurred vision with Ivermectin
- Endpoint of negative PCR is not reflective of disease as patient can be fully recovered but still have enough viral material to be amplified by PCR (ie irrelevant viral material)
- Bottom Line: Like many of the other studies reviewed on this post, the findings are interesting, however a RCT of 24 healthy patients, without comorbid disease, with no improvements in patient-oriented outcomes (i.e. mortality, progression of disease) does not justify early adoption of Ivermectin.
Evidence from India [9]
What They Did:
- Observational study of 118 healthcare providers in Bangladesh
- Subjects divided into:
- Experimental: Monthly Ivermectin 12mg x4months
- Control: No treatment
Outcomes:
- Acquiring COVID-19
Inclusion:
- Healthcare workers working in COVID-19 isolation wards
- Age 21 to 60 years
- No treatment with any antiviral drugs
Exclusion:
- ≥60 years of age and <21 years of age
- Pregnant women or lactating mothers
- Chronic liver disease
- Symptomatically ill
Results:
- 118 healthcare workers
- COVID-19 Diagnosis:
- Experimental: 4/58 (6.9%)
- Control: 44/60 (73.3%)
- P <0.05
Discussion:
- INTERESTING FACT:Ivermectin has a plasma half-life of ≈16 to 18hrs with time-length ranging from 4 to 12days. The reason this is interesting is that if Ivermectin is only given 1x/month you wouldn’t have effective drug in your system after 12 days. This makes a 1x/month dosing scheme pharmacologically irrelevant for the finding
- A 70% conversion rate is ridiculously high. This makes me question how good PPE use and availability were during this trial
Bottom Line: Of all the studies reviewed in this post, this is the most promising. However it’s a single center study, observational and there are a number of factors that are unknown. Given the high conversion rate in the control group, it would be important to know what level of PPE was being used and if it was different between groups. Additionally, there was no patient oriented outcomes relegating the results of this study to promising and hypothesis generating requiring larger RCTs to confirm the results.
The EPIC Trial – RCT in Colombia [10]
Clinical Question: What is the effect of ivermectin on duration of symptoms in adults with mild COVID-19?
What They Did:
- Double-blind, randomized trial conducted at a single center in Colombia
- Patients were identified by random sampling from the state’s health department electronic database
- Patients randomized 1:1 to:
- Ivermectin 300ug/kg per day x5d
- Matching placebo per day x5d
Outcomes:
- Primary:Time to resolution of symptoms within 21d
Results:
- 476 adult patients with mild disease included
- 400 patients randomized in the primary analysis
- Median age = 37 years
- 79% did not have comorbid conditions at baseline
- 398 (99.5%) completed the trial
- No difference in adverse events or serious adverse events between groups
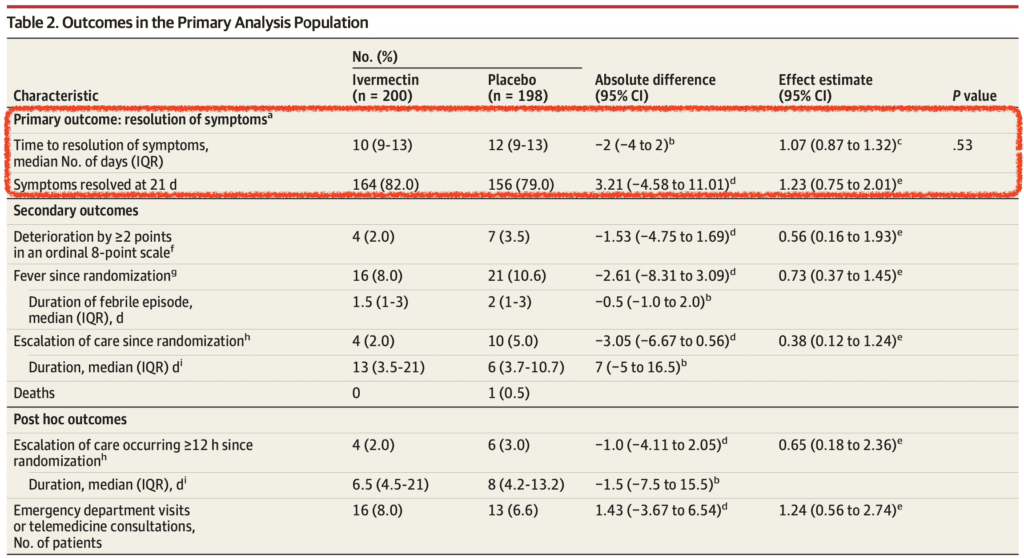
Discussion:
- Young patient population without comorbid disease does not allow for extrapolation of results to older patients and/or patients with comorbid conditions
- Up to August 26th, 2020 placebo was a mixture of 5% dextrose in saline and dextrose in distilled water. Due to potential for unblinding due to different taste and smell of ivermectin vs saline/dextrose placebo only 1 patient per household was included in the study to minimize chances of unblinding
- Collected bottles at the end of study to certify adherence to the assigned regimen
- Original primary outcome was defined as the time from randomization until worsening 2 points on the 8-category ordinal scale. Before the interim analysis, it became apparent that the pooled event rate of worsening by 2 points was substantially lower than the expected 18%, creating an unattainable sample size. Therefore, on Aug 31st, 2020 the primary outcome was changed to time from randomization to complete resolution of symptoms within 21d
- On Oct. 20th, 2020 it was realized that a labeling error occurred between Sept 29thand Oct 15th, 2020 resulting in all patients receiving ivermectin and none receiving placebo during this time frame. Study blinding was not unmasked due to this error and these patients were excluded from the primary analysis
- Authors used daily instead of intermittent dosing of ivermectin based on pharmacokinetic models showing higher lung concentrations with daily dosing
- Although there was a numerically smaller group of patients in the ivermectin arm that required escalation of care vs placebo (2.0% vs 5.0%), this difference was not statistically significant and after removing 4 patients that were hospitalized within 3.25hrs after randomization this finding was further diminished
- Additionally, ivermectin did not reduce ED visits or telephone consultations compared to placebo in this trial
- Bottom Line: This randomized, double-blinded, placebo-controlled trial showed no benefit to the use of ivermectin compared to placebo in resolution of symptoms by 21 days. The methodology of this trial is certainly of better quality than previous trials, however there are some clear short comings with errors and potential for unblinding. Also, the relatively young and healthy study population included in this trial makes it difficult to extrapolate conclusions to older patient populations and/or patients with comorbid disease. We will just have to wait and see what future trials in different patient populations show.
Cochrane Review (Link is HERE)
Conclusion: “We found no evidence to support the use of ivermectin for treating or preventing COVID-19 infection, but the evidence base is limited.”
Discussion:
- All of these trials discuss the efficacy of Ivermectin in the treatment of COVID-19 at various time points in illness, but very little was mentioned about safety
- Due to its massive global use in low- and middle-income countries, the knowledge base establishing a high margin of safety and low rate of adverse effects is nearly unparalleled
- Most common adverse events are mild and transient
- Adverse effects are likely attributed to bodies inflammatory response and include itching, rash, swollen lymph nodes, joint pain, fever, and headache
- In one trial of 17,877 patients treated with ivermectin 150mcg/kg for Loa loa in Cameroon. Only 20 patients (0.11%) developed serious reactions without neurological signs lasting for more than a week
-
The definition of insanity: Doing the same thing over and over again and expecting different results
- We have seen the adoption of therapeutics too early during this pandemic with many of them not panning out with subsequent RCTs
- Hydroxychloroquine is a perfect example of this:
- In vitro studies showing decrease in viral load
- Subsequent observational trials showing associations of benefit
- Later randomized clinical trials showing no benefit and potential harms
- If you believe the evidence thus far, which are severely flawed: Dosing of Ivermectin Used in Trials
- Prophylaxis: 0.2 mg/kg on day 1 and day 3 followed by one dose/month
- Treatment 0.2mg/kg o day 1 and day 3 followed by Days 6 and 8 if not recovered
Statement From Merck (Maker of Ivermectin) [Link is HERE]

Clinical Take Home Point:
- There is no “High-quality” evidence showing that ivermectin plays a role in treatment of COVID19.Unless current ongoing RCTs show a benefit, ivermectin should only be used in the setting of a clinical study
- Evidence for the use of Ivermectin is based on in vitro, prophylaxis, clinical, safety, and large-scale epidemiologic studies (heterogenous populations in multiple different settings) BUT…
- Many of the trials thus far are methodologically flawed without enough information about baseline demographics, multiple primary outcomes, soft/subjective outcomes, convenience samples, and unclear definitions, just to name a few
- Additionally, a valid concern in evaluating the literature is that many of the trials have not yet passed the peer review process and are in pre-print format
- Although Ivermectin is cheap, readily available, with a fairly safe side effect profile, based on the evaluation of the literature above, at this time, Ivermectin should not be recommended outside of a clinical trial to ensure we get a true answer of effect
- Ivermectin is interesting, there is certainly signal to evaluate further, but in our desire to want a treatment option, let’s not continue to do the same thing over and over again, as we saw play out with Hydroxychloroquine
References:
- Kory P et al. Review of the Emerging Evidence Demonstrating the Efficacy of Ivermectin in the Prophylaxis and Treatment of COVID-19. FLCCC Alliance 2020. [Link is HERE]
- Caly L et al. The FDA-Approved Drug Ivermectin Inhibits the Replication of SARS-CoV-2 in Vitro. Antiviral Res 2020. PMID: 32251768
- Elgazzar A et al. Efficacy and Safety of Ivermectin for Treatment and Prophylaxis of COVID-19 Pandemic. Research Square 2020 Pre-Print [Link is HERE]
- Hellwig MD et al. A COVID-19 Prophylaxis? Lower Incidence Associated with Prophylactic Administration of Ivermectin, International Journal of Antibcrobial Agents 2020. PMID: 33259913
- Hashim A et al. Controlled Randomized Clinical Trial on Using Ivermectin with Doxycycline for Treating COVID-19 Patients in Baghdad, Iraq. medRxiv PrePrint 2020 [Link is HERE]
- Gardon J et al. Serious Reactions After Mass Treatment of Onchocerciasis with Ivermectin in an Area Endemic for Loa Loa Infection. Lancet 1997. PMID: 9217715
- Rajter JC et al. Use of Ivermectin is Associated with Lower Mortality in Hospitalized Patients with Coronavirus Disease 2019: The ICON Study. CHEST 2020. PMID: 33065103
- Chaccour C et al. The Effect of Early Treatment with Ivermectin on Viral Load, Symptoms and Humoral Response in Patients with Non-Severe COVID-19: A Pilot, Double-Blind, Placebo Controlled, Randomized Clinical Trial. Lancet 2021 [Link is HERE]
- Alam MT et al. Ivermectin as Pre-Exposure Prophylaxis for COVID-19 Among Healthcare Providers in a Selected Tertiary Hospital in Dhaka – An Observational Study. EJMED 2020. [Link is HERE]
- Lopez-Medina E et al. Effect of Ivermectin on Time to Resolution of Symptoms Among Adults with Mild COVID-19: A Randomized Clinical Trial. JAMA 2021. PMID: 33662102
- Samaha AA et al. Effects of a Single Dose of Ivermectin on Viral and Clinical Outcomes in Asymptomatic SARS-CoV-2 Infected Subjects: A Pilot Clinical Trial in Lebanon. Viruses 2021. PMID: 34073401
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post COVID-19 Update: Ivermectin appeared first on REBEL EM - Emergency Medicine Blog.