
 Background: Despite limited high-quality evidence, many institutions are currently using convalescent plasma therapy (CPT) in the treatment of COVID-19. The majority of the evidence for CPT comes from observational studies lacking placebo arms (US Expanded Access Program). Convalescent plasma therapy isn’t a novel treatment modality and has been used in the treatment of other infectious diseases (SARS, MERS, H1N1, Ebola, etc…) with mixed results. The theory behind CPT is it can supplement the patient’s immune response by administering plasma rich in antibodies from someone previously infected who has recovered. Thus far the US Expanded Access Program, showed that giving convalescent plasma earlier (i.e. ≤3 days) and with higher titers (>18.45 S/Co) was associated with improved mortality in COVID-19 (This data cannot show causality as there was no randomization and no control arm) [3].
Background: Despite limited high-quality evidence, many institutions are currently using convalescent plasma therapy (CPT) in the treatment of COVID-19. The majority of the evidence for CPT comes from observational studies lacking placebo arms (US Expanded Access Program). Convalescent plasma therapy isn’t a novel treatment modality and has been used in the treatment of other infectious diseases (SARS, MERS, H1N1, Ebola, etc…) with mixed results. The theory behind CPT is it can supplement the patient’s immune response by administering plasma rich in antibodies from someone previously infected who has recovered. Thus far the US Expanded Access Program, showed that giving convalescent plasma earlier (i.e. ≤3 days) and with higher titers (>18.45 S/Co) was associated with improved mortality in COVID-19 (This data cannot show causality as there was no randomization and no control arm) [3].
Paper: Simonovich VA et al. A Randomized Trial of Convalescent Plasma in COVID-19 Severe Pneumonia. NEJM 2020. PMID: 33232588
Clinical Question: Is convalescent plasma effective in the treatment of COVID-19 pneumonia compared to placebo?
What They Did:
- Double-blind, placebo-controlled, multicenter, randomized controlled trial of hospitalized adult patients with severe COVID-19 pneumonia
- Conducted at 12 clinical sites in Argentina
- Patients randomized in a 2:1 ratio to receive:
- Single dose convalescent Plasma (CP): Median titer of 1:3200 (Range 1:800 to 1:3200)
- Placebo (P): 0.9% saline solution
- Everyone in both groups received standard treatment
- This included antiviral agents, glucocorticoids, or both
Outcomes:
-
Primary: Clinical status 30 days after intervention, measured on a 6-point ordinal scale ranging from total recovery to death
- 1 = death
- 2 = invasive ventilatory support
- 3 = hospitalized with supplemental O2 requirement
- 4 = hospitalized without supplemental O2
- 5 = discharged without full return to baseline physical function
- 6 = discharged with full return to baseline physical function
-
Secondary:
- Clinical status on the ordinal scale at days 7, 14, and at the time (in days) to:
- Discharge from the hospital
- Time to discharge from the ICU
- Time to improvement in at least two categories on the ordinal scale
- Time to death
- Time to full functional recovery
- Incidence of adverse and serious adverse events
- Clinical status on the ordinal scale at days 7, 14, and at the time (in days) to:
Inclusion:
- Hospitalized adult (≥18 years) patients
- RT-PCR positive for COVID-19
- Radiologically confirmed PNA
- No previous directives rejecting advanced live support
- At least one of the following:
- SaO2 <93% on RA
- P/F ratio <300mmHg
- SOFA or mSOFA score ≥2 above baseline status (Scores range from 0 to 24 with higher scores indicating more severe disease)
Exclusion:
- Pregnant or lactating
- Reproductive age not willing to use contraceptive measures for a period of 30d after enrollment
- History of blood component allergies
- Infectious cause of PNA other than SARS-CoV-2
- Requirement for mechanical ventilation
- Multiorgan failure
- Unable to provide informed consent
Results:
- 448 patients were assessed for eligibility
- 334 underwent randomization
- 228 patients assigned to receive CP
- 105 patients assigned to receive P
- Median time from onset of symptoms to enrollment was 8 days (Range 5 to 10d)
- Hypoxemia was the most frequent criterion for enrollment (≈97%)
- Use of glucocorticoids in ≈93% of patients
- Almost no patients treated with Lopinavir-ritonavir, Tocilizumab, Ivermectin, or Hydroxychloroquine
- Of the 215 patients from whom a baseline anti-SARS-Cov-2 IgG antibody level could be obtained the median titer was 1:50 (46.0% of patients had no detectable antibody level)
-
No difference in the distribution of clinical status at 30d after intervention (Primary Outcome)
- OR 0.83; 95% CI 0.52 to 1.35; p = 0.46
- After balancing for sex, history of COPD, and history of tobacco use OR 0.92; 95% CI 0.59 to 1.42; p = 0.70
- An OR >1.0 would correspond to a more favorable outcome
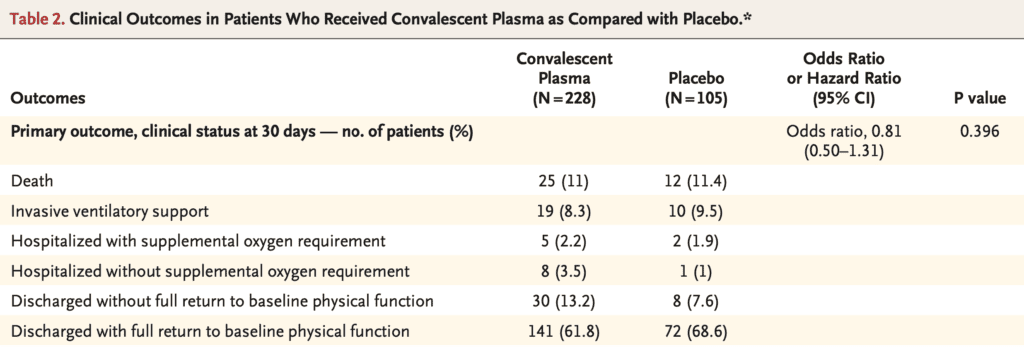
- Overall Mortality:
- CP: 10.96%
- P: 11.43%
- Risk Difference: -0.46%; 95% CI -7.8 to 6.8
- SARS-CoV-2 antibody titers tended to be higher in the CP group at day 2 after intervention
- No differences in:
- Clinical status on ordinal scale at day 7 or day 14
- ICU admissions (CP 53.9% vs P 60%)
- Invasive ventilatory support (CP 26.8% vs P 22.9%)
- Median time from intervention to hospital DC, DC from ICU, complete restoration of physical functions, to start of invasive ventilation or to death
- Adverse events and serious adverse events were similar between groups with the exception of sex, history of COPD and history of tobacco use
- Infusion-related adverse events were slightly more common in the CP group (4.8%) vs P group (1.9%) OR 2.62; 95% CI 0.57 to 12.04)
Strengths:
- Study asks an important clinical question with a patient centered outcome
- First double-blind trial of CPT
- No patients were lost to follow up
- Entire clinical team, data collectors, and outcome adjudicators were unaware of the treatment assignments
- Authors ensured that transfused CP had a total anti-SARS-CoV-2 antibody titer of at least 1:800 and the plasma volume infused had a correction factor according to patient’s weight
- Had a number of prespecified subgroup analyses in an attempt to detect patient subgroups for which convalescent plasma may have had a potential benefit
Limitations:
- All patients enrolled had “severe” COVID-19 pneumonia, therefore results cannot be extrapolated to other groups (i.e. mild to moderate cases)
- The median time from symptom onset to enrollment was 8 days in this trial. Therefore, no firm conclusion can be drawn regarding efficacy of passive immune therapy earlier than the median time
- Usual therapy was not standardized among participating sites
- Unclear if there was a consecutive enrollment of patients and it appears based on the numbers over the period of time, that this was a convenience sample
- IMPORTANT: Post infusion reactions such as transfusion-associated cardiac overload (TACO) and transfusion-related acute lung injury (TRALI) could not be differentiated from COVID-19 progression in the spectrum of patients included in this trial
Discussion:
- The PLACID trial [2], another RCT of convalescent plasma in patients with moderate COVID-19 also showed no difference in progression of disease or death at day 30. The biggest issues with this trial are that it was open-label (i.e. not blinded) and the infused convalescent plasma had very low titers of anti-SARS-CoV-2 antibodies
- Although there was an increase in antibody titers for SARS-CoV-2 by day 2 in the convalescent arm compared to the placebo arm, these higher titers were diluted later in the trial (This is an expected result)
- In the subgroup of patients <65 years of age, there was a difference in the primary outcome, however the difference favored placebo (OR 0.18; 95% CI 0.06 to 0.54). This could be a chance finding, but warrants further research to confirm or refute this finding
Author Conclusion: “No significant differences were observed in clinical status or overall mortality between patients treated with convalescent plasma and those who received placebo.”
Clinical Take Home Point: Convalescent plasma therapy (CPT) in patients with severe pneumonia due to COVID-19 did not reduce mortality or improve other clinical outcomes at day 30 compared to placebo. Based on the results of this trial and the PLACID RCT, CPT should not be standard care in patients with COVID-19 pneumonia.
References:
- Simonovich VA et al. A Randomized Trial of Convalescent Plasma in COVID-19 Severe Pneumonia. NEJM 2020. PMID: 33232588
- Agarwal A et al. Convalescent Plasma in the Management of Moderate COVID-19 in Adults in India: Open-Label Phase II Multicentre Randomised Controlled Trial (PLACID Trial). BMJ 2020. PMID: 33093056
- Joyner MJ et al. Effect of Convalescent Plasma on Mortality Among Hospitalized Patients with COVID-19: Initial Three-Month Experience. medRxiv 2020. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- REBEL EM: US Expanded Access Program – Convalescent Plasma, Mortality, & COVID-19
- The Bottom Line: PlasmAr
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post COVID-19 Update: The PlasmAr Trial – Convalescent Plasma vs Placebo appeared first on REBEL EM - Emergency Medicine Blog.


