 Background: Evidence from the CRASH-2 trial showed an absolute reduction in mortality of 1.5% (NNT = 67) in patients with extracranial bleeding treated with tranexamic acid (TXA) within 3 hours of injury. However, CRASH-2 did not answer the question of effect on mortality in patients with intracranial hemorrhage (ICH), as these patients were excluded from the trial. It makes biologic sense that administration of TXA in patients with traumatic brain injury (TBI) might prevent or reduce ICH expansion and thus avert brain herniation and death. There were two smaller RCTs [2] that showed a reduction in death with TXA in patients with ICH. However, both of these trials were small and considered to be hypothesis generating only. TICH-2 [3] was an international, randomized, double-blind, placebo-controlled phase 3 trial in adults with ICH from acute stroke with ≈2300 patients and showed no difference between groups in functional status at day 90. TICH-2 did show a small improvement in hematoma expansion at day 2 and death by day 7. Due to the fact that these findings were secondary outcomes they were also hypothesis generating. All of the above positive findings therefore required confirmation in a larger randomized trial, which has finally arrived…CRASH-3.
Background: Evidence from the CRASH-2 trial showed an absolute reduction in mortality of 1.5% (NNT = 67) in patients with extracranial bleeding treated with tranexamic acid (TXA) within 3 hours of injury. However, CRASH-2 did not answer the question of effect on mortality in patients with intracranial hemorrhage (ICH), as these patients were excluded from the trial. It makes biologic sense that administration of TXA in patients with traumatic brain injury (TBI) might prevent or reduce ICH expansion and thus avert brain herniation and death. There were two smaller RCTs [2] that showed a reduction in death with TXA in patients with ICH. However, both of these trials were small and considered to be hypothesis generating only. TICH-2 [3] was an international, randomized, double-blind, placebo-controlled phase 3 trial in adults with ICH from acute stroke with ≈2300 patients and showed no difference between groups in functional status at day 90. TICH-2 did show a small improvement in hematoma expansion at day 2 and death by day 7. Due to the fact that these findings were secondary outcomes they were also hypothesis generating. All of the above positive findings therefore required confirmation in a larger randomized trial, which has finally arrived…CRASH-3.
What They Did:
- Randomized, placebo-controlled trial done in 175 hospitals in 29 countries
- Adults with TBI who were within 3 hrs of injury, had a GCS score of ≤12 or any intracranial bleeding on CT scan, and no major extracranial bleeding were eligible (The original protocol had eligibility listed as 8hr but in 2016 the protocol was changed to limit recruitment to patients to ≤3hrs from injury)
- Patients randomly assigned to receive tranexamic acid (loading dose 1g over 10 min then infusion of 1g over 8hr) or matching placebo (0.9% normal saline)
Outcomes:
- Primary: Head injury-related death in hospital within 28 days of injury in patients treated within 3 hours of injury
- Secondary:
- Early head injury-related death (within 24h after injury)
- All-cause and cause specific mortality
- Disability
- Vascular occlusive events (MI, CVA, DVT, and PE)
- Seizures
- Complications
- Neurosurgery
- Days in ICU
- Adverse events within 28d of randomization
Inclusion:
- Adults with TBI within 3h of injury (Time window was originally 8h of injury, however in 2016 the protocol was amended to ≤3hrs of injury based on best available evidence)
- GCS ≤12
- Any intracranial bleeding on CT scan
- No major extracranial bleeding
Exclusion:
- <16 years of age
- Extracranial bleeding
- >8hrs since injury (limited to >3hrs from Sept 2016)
- Prespecified sensitivity analysis excluding patients with GCS of 3 and those with bilateral non-reactive pupils
Results:
- Randomly allocated 12,737 patients with TBI
- TXA group: 6,406
- Placebo group: 6,331
- Of the 12,737 patients 9,202 (72.2%) were treated within 3hr of injury
- Mortality by Days from Injury
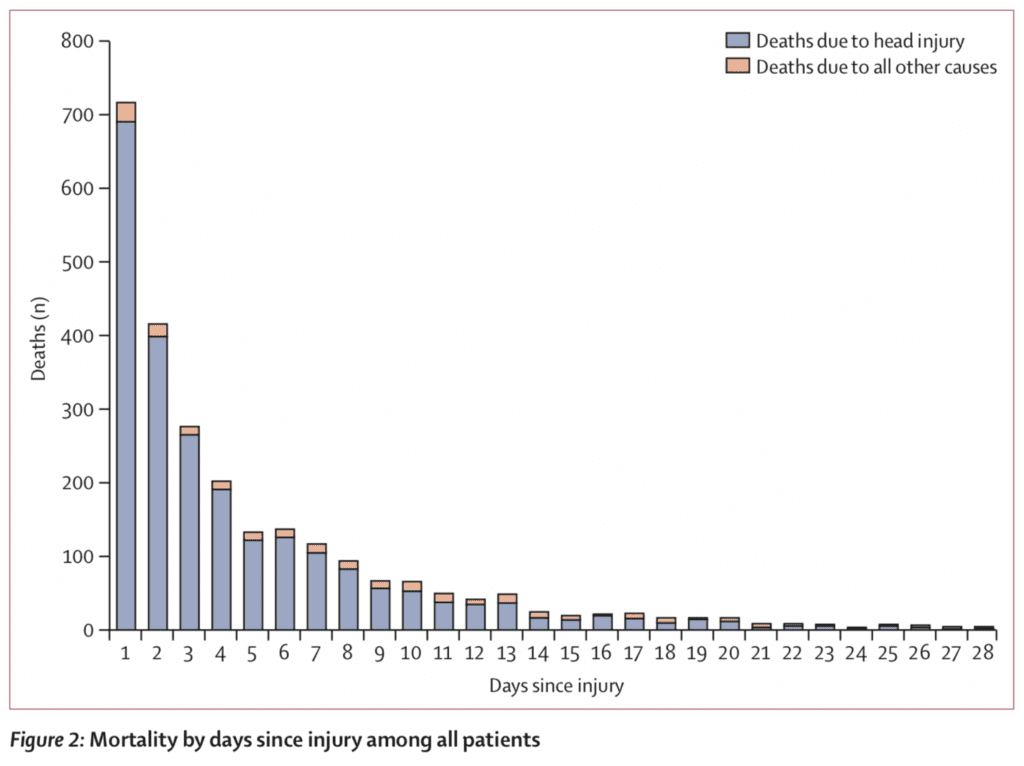
-
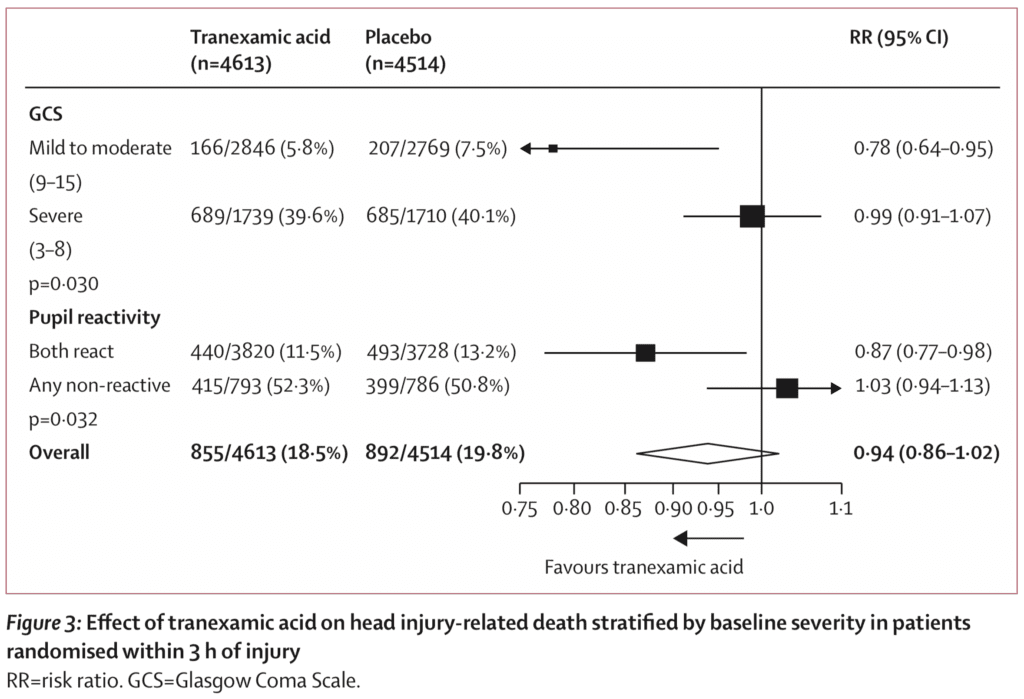
Risk of head injury-related death within 3hrs from injury (Primary Outcome):
- TXA: 18.5%
- Placebo: 19.8%
- RR 0.94
- 95% CI 0.86 – 1.02
- Not statistically significant
- Prespecified sensitivity analysis removing patients with a GCS of 3 and bilateral unreactive pupils at baseline:
- TXA: 12.5%
- Placebo: 14.0%
- RR 0.89
- 95% CI 0.80 – 1.00
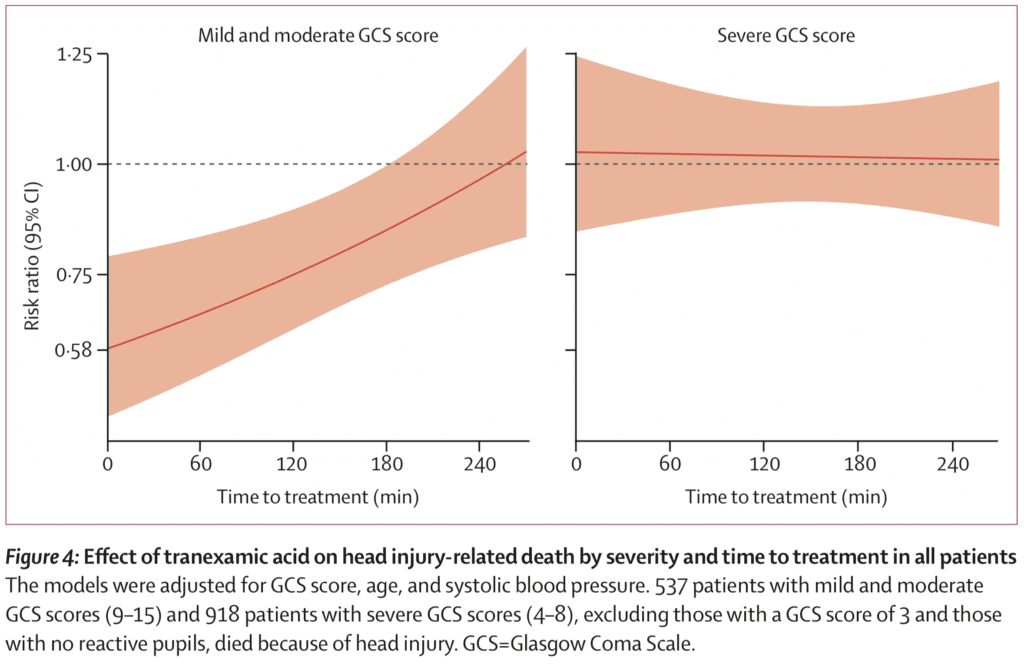
- Risk of head injury-related death in patients with mild-to-moderate head injury (GCS 9 – 15) was reduced (RR 0.78; 95% CI 0.64 – 0.95) but not in patients with severe head injury (GCS 3 – 8) (RR 0.99; 95% CI 0.91 – 1.07)
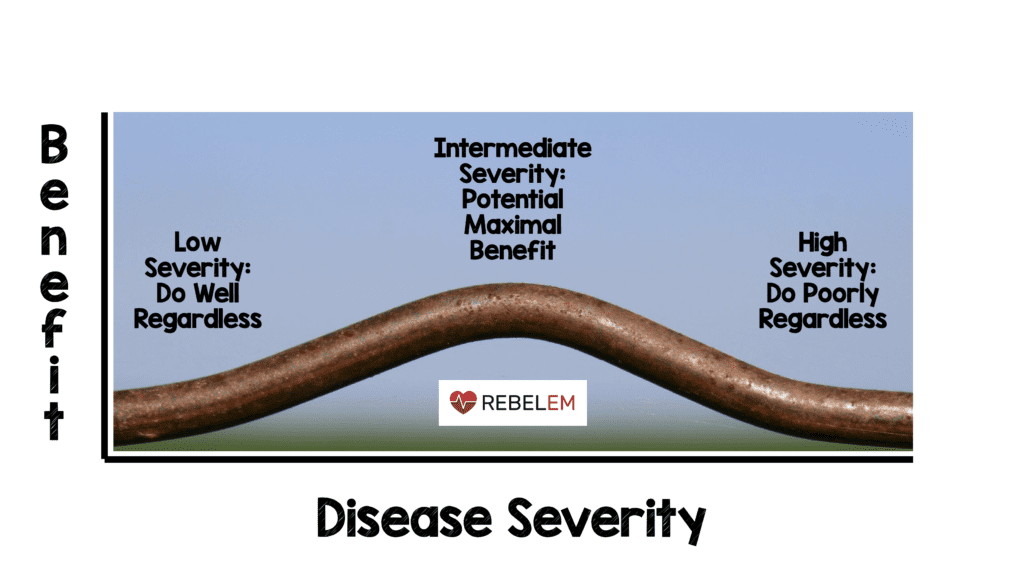
- When baseline GCS was used in a regression analysis found evidence that TXA is more effective in less severely injured patients (p = 0.007) and among patients with reactive pupils head injury-related deaths were reduced with TXA (0.87; 95% CI 0.77 – 0.98)
- Effect of TXA on disability in survivors by comparing mean Disability Rating Scale (Lower means less disabled) in patients treated within 3h of injury:
- TXA: 4.99
- Placebo: 5.03
- Not statistically significant
- Risk of vascular occlusive events was similar in both groups (RR 0.98; 95% CI 0.74 – 1.28)
Strengths:
- Asks a clinically important question
- Largest RCT to assess the utility of IV TXA in ICH
- Blinding and randomization were appropriately performed
- Patients, caregivers, and those assessing outcomes were masked to allocation
- Used an intention-to-treat principle which assesses clinical effectiveness that mirrors actual practice, when not everyone adheres to the treatment. This type of analysis avoids the effects of crossover and dropout
- The authors clearly explained why they changed their protocol from 3 to 8 hours and that they changed their power analysis and number needed based on this as well
- Prespecified sensitivity analysis excluded patients with patients with a GCS of 3 and bilateral non-reactive pupils at baseline removing patients with poor prognosis for neurologic outcomes
- Packaging was identical in appearance between TXA and placebo
- Small number of patients lost to follow-up (38 and 33), had withdrawn consent (16 and 24), or did not have outcome data available (9 and 18)
- Baseline characteristics were similar between treatment groups
- Almost all patients with TBI who met inclusion criteria were recruited into the study with almost no loss to follow up
- Anticipated that patients with TBI and a GCS of 3 and those with bilateral unreactive pupils before treatment would have little potential benefit from TXA
Limitations:
- Originally planned for 10,000 patients to have a 90% powered to detect a 15% relative reduction in mortality, however with the change in the primary outcome to head injury-related death in hospital within 28d of injury in patients randomly assigned within 3h of injury the sample size was increased to 13,000 to have approximately 10,000 patients treated within 3hr of injury which they did not meet (≈9,200 patients)
- Wide confidence intervals despite having a large number of patients
- Authors had to narrow down the group to get to a significant benefit and this decreases external validity and makes it more difficult to apply and certainly means it doesn’t apply to all TBI patients
- This was not a hemodynamically unwell patient population with 97% of patients having SBPs ≥90mmHg which maybe one of the reasons we do not see a larger beneficial effect of TXA vs placebo
- PE and DVT only diagnosed when found positive on imaging or post-mortem examination which might underestimate the risk of DVT or PE
- Patients with unilateral non-reactive pupils were not excluded and for many of these patients, there may have already been some brain herniation and diluted the treatment effect toward the null
Discussion:
- The authors pooled all results from previous and current evidence of TXA in ICH and found an overall mortality benefit when assessing all the available RCT evidence of TXA increasing the possibility of mortality benefit
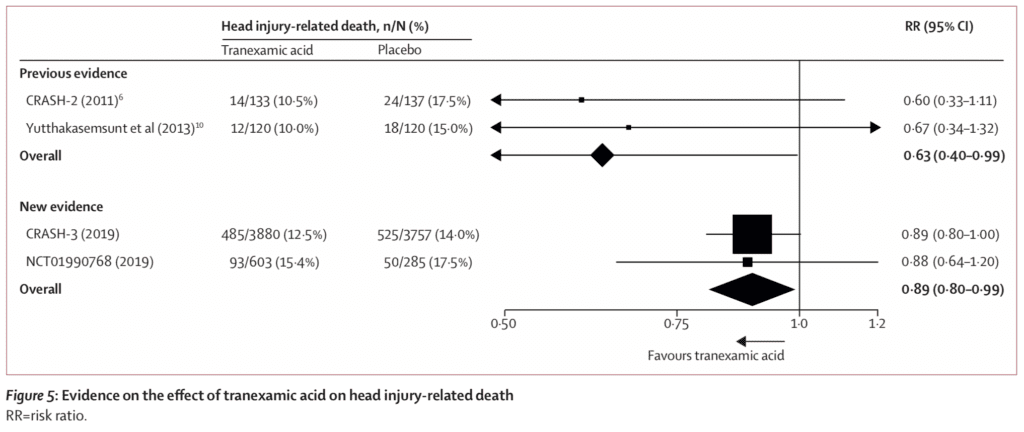
- The data from this trial support the hypothesis, showing a reduction in head injury-related deaths within 24h of injury, which were similar to the results obtained from the CRASH-2 trial of TXA in patients with traumatic extracranial bleeding. After 24 hours the benefit of TXA in head injury patients was attenuated, most likely due to the fact that patients died due to non-bleeding related mechanisms
- When the effect of TXA on head injury-related death was stratified by time to treatment of ≤1hr, 1hr to 3hr, and >3hr there was no statistically different outcome. However patients treated soon after injury often had more severe head injury so the effect of time to treatment could be confounded by severity…As stated above early treatment was more effective than later treatment in patients with mild and moderate head injury (p = 0.005)
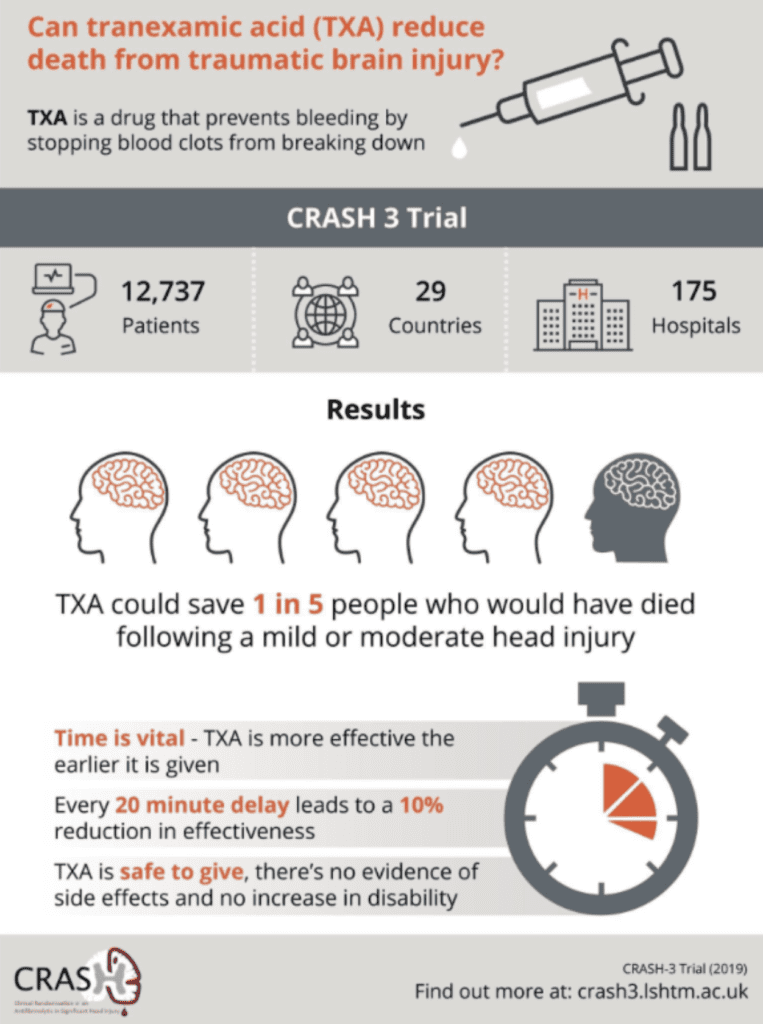
- Interestingly the trial authors put an infographic after the publication of this trial that had a very positive spin on the results of the trial. Ken Milne from the Skeptics Guide to Emergency Medicine had a more skeptical view of the infographic and made some corrections which I believe to be more spot on.
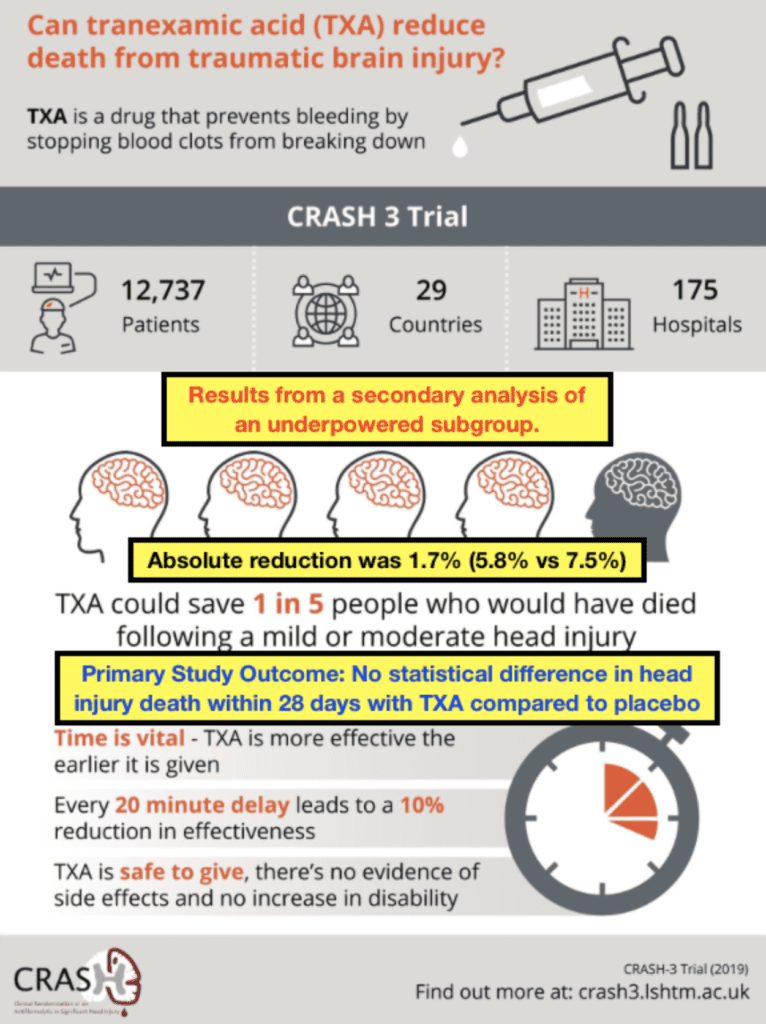
Author Conclusion: “Our results show that tranexamic acid is safe in patients with TBI and that treatment within 3 h of injury reduces head injury-related death. Patients should be treated as soon as possible after injury.”
Clinical Take Home Point: The overall effect size of TXA on ICH is not that impressive based on this trial and also not statistically significant. It is possible that the effect of TXA on head injury-related death is dependent on the time interval between injury and the severity of the TBI. However, this is a select group of patients defined as:
- Early treatment (<3hrs)
- Mild to moderate (GCS 9 – 15)
- ICH on baseline head CT.
These patients potentially have the highest mortality benefit, and it would be reasonable to give TXA, but, would not consider it standard practice at this point in time based on the best available evidence. Given there were minimal adverse events, it would be wrong to conclude that TXA in ICH is ineffective as there are patients who may improve with this therapy, however with wide confidence intervals, it is difficult to make definitive conclusions when looking at these subpopulations.
References:
- The CRASH-3 Trial Collaborators. Effects of Tranexamic Acid on Death, Disability, Vascular Occlusive Events and Other Morbidities in Patients with Acute Traumatic Brain Injury (CRASH-3): A Randomised, Placebo-Controlled Trial. Lancet 2019. [Epub Ahead of Print HERE]
- Zehtabchi S et al. Tranexamic Acid for Traumatic Brain Injury: A Systematic Review and Meta-Analysis. Am J Emerg Med 2014. PMID: 25447601
- Sprigg N et al. Tranexamic Acid for Hyperacute Primary IntraCerebral Haemorrhage (TICH-2): An International Randomised, Placebo-Controlled, Phase 3 Superiority Trial. Lancet 2018. PMID: 29778325
For More Thoughts on This Topic Checkout:
- St. Emlyn’s: JC – Tranexamic Acid (TXA) in Head Injury. The CRASH-3 Results
- The SGEM: SGEM#270 – CRASH-3 TXA fro Traumatic Head Bleeds
- PulmCrit: Tranexamic Acid For Traumatic Brain Injury (CRASH3)
- EMNerd: The Case of the Indecisive Antidote
- EMLit of Note: CRASH-3!
- The Resus Room: CRASH-3
- BadEM: CRASH 3
- The BREACH: CRASH-3 – Tranexamic Acid for Intracranial Hemorrhage
- Journal Feed: CRASH-3 – TXA for TBI
- First10EM: CRASH3 – TXA is No Wonder Drug
- The Bottom Line: CRASH-3
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post CRASH-3: TXA for ICH? appeared first on REBEL EM - Emergency Medicine Blog.