
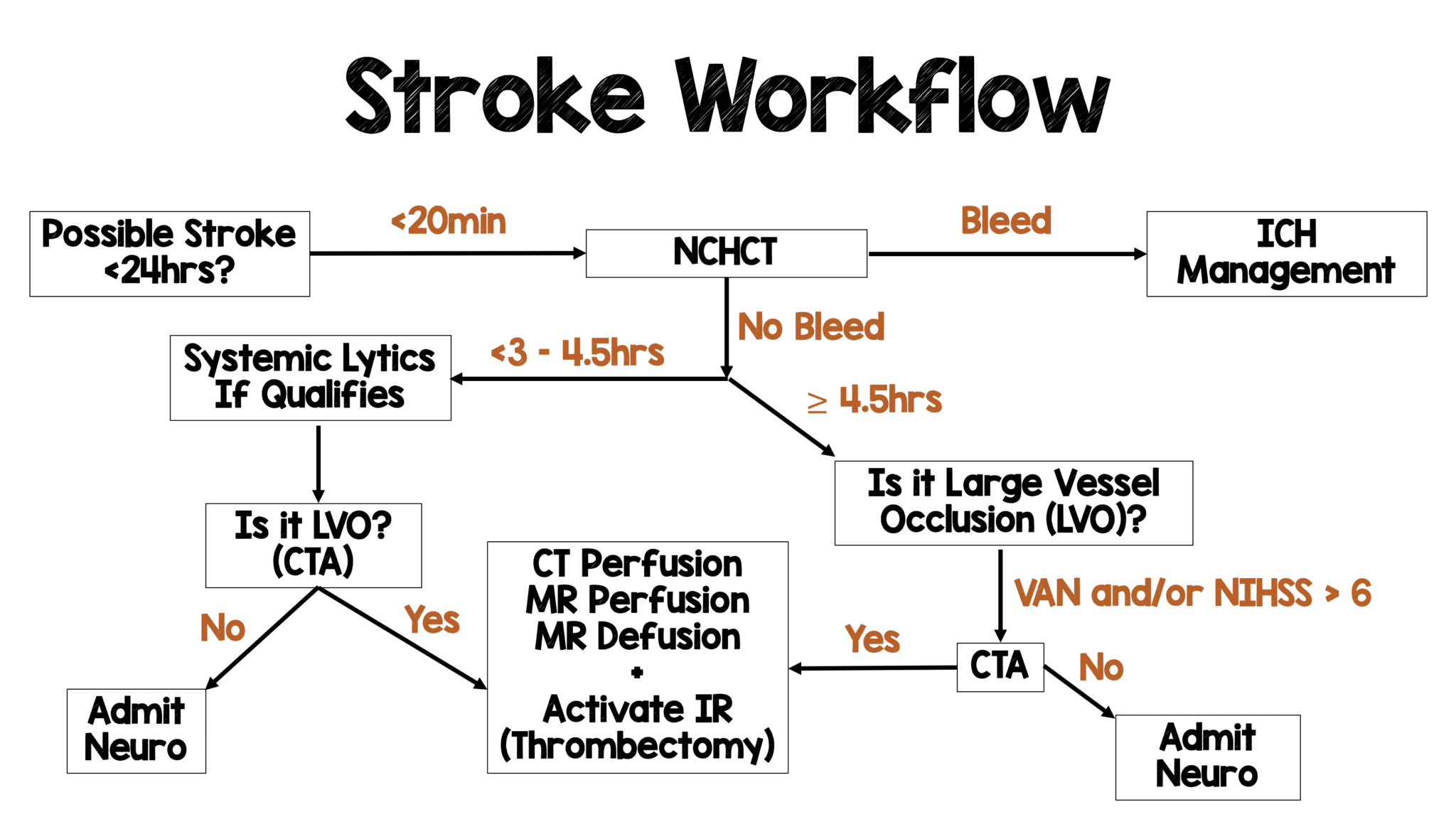
 Background: The publication of the MR CLEAN trial in January 2015 changed the face of ischemic stroke care. This was the first study demonstrating a benefit to endovascular treatment of a specific subset of ischemic stroke patients: those with a large vessel occlusion (LVO) presenting within 6 hours of symptom onset. MR CLEAN was followed by a flurry of publications seeking to replicate and refine treatment as well as expand the window for treatment. The REBEL team reviewed this literature back in 2018 and, with the help of Dr. Evie Marcolini, created the below workflow:
Background: The publication of the MR CLEAN trial in January 2015 changed the face of ischemic stroke care. This was the first study demonstrating a benefit to endovascular treatment of a specific subset of ischemic stroke patients: those with a large vessel occlusion (LVO) presenting within 6 hours of symptom onset. MR CLEAN was followed by a flurry of publications seeking to replicate and refine treatment as well as expand the window for treatment. The REBEL team reviewed this literature back in 2018 and, with the help of Dr. Evie Marcolini, created the below workflow:
One major component of LVO management is the use of systemic thrombolytics in patients presenting within the current thrombolytic treatment window prior to endovascular intervention. However, there is no data to guide clinicians on the dose of thrombolytics in this circumstance. In fact, there is no high-quality data to support the role for systemic thrombolytics and, there is limited evidence that it does not improve outcomes (Phan 2017, Rai 2018). Adding more controversy to the matter is the question of thrombolytic selection (ie alteplase or tenecteplase). While the majority of the literature is around alteplase, tenecteplase has some benefits including ease of use (bolus administered over 5 seconds as opposed to alteplase which requires 10% to be given as a bolus and the other 90% administered over an hour). The ideal dose of tenecteplase for this indication is unknown with studies looking at 0.10 mg/kg, 0.25 mg/kg as well as 0.4 mg/kg. There is a clear need for further research into systemic thrombolytic dosing and use.
Article: Campbell BCV et al. Effect of intravenous tenecteplase dose on cerebral repercussion before thrombectomy in patient with large vessel occlusion ischemic stroke: The EXTEND-IA TNK Part 2 Randomized Clinical Trial. JAMA 2020. PMID: 32078683
Clinical Question: Does higher dose tenecteplase (0.40 mg/kg) result in improved cerebral reperfusion when administered prior to endovascular therapy in LVO ischemic stroke?
Population: Adult patients (18 years of age) presenting within 4.5 hours of ischemic stroke symptom onset (and eligible for systemic thrombolytics) and with cerebral vascular occlusion on CT angiography of the intracranial internal carotid artery, middle cerebral artery (first or second segments) or basilar artery and if endovascular thrombectomy was intended to be performed (groin puncture within 6hrs of stroke onset).
Outcomes:
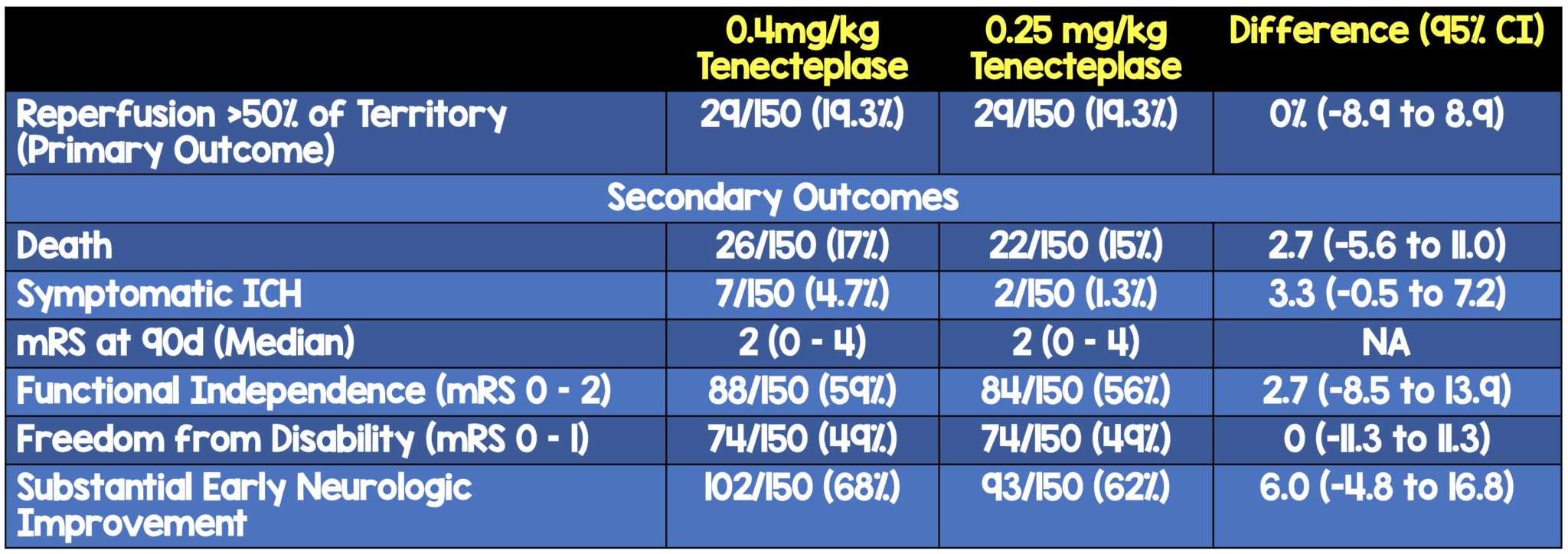
- Primary: Reperfusion > 50% of the involved ischemic territory prior to endovascular therapy (EVT) or an absence of retrievable intracranial thrombus.
-
Secondary:
- Level of disability at 90 days
- Freedom from disability = mRS score of 0 to 1 (or no change from baseline) at 90 days
- Functional independence = mRS 0-2 (or no change from baseline) at 90 days
- Substantial early neurological deficit improvement at day 3
- Defined reduction of NIHSS of at least 8 points (or reaching 0-1) at day 3
- All-cause death
- Symptomatic intracranial hemorrhage within 36hrs
Intervention: 0.40 mg/kg (maximum of 40 mg) IV tenecteplase given as a bolus
Control: 0.25 mg/kg (maximum of 25 mg) IV tenecteplase given as a bolus
Design: Multicenter, open-label, randomized clinical trial across 27 hospitals in Australia and 1 in New Zealand
Excluded:
- Patients with severe premorbid disability (mRS > 3)
- Extensive NCHCT hypodensity (>1/3 of MCA or basilar artery territory)
- Intracranial hemorrhage on CT or MRI
- Rapidly improving symptoms
- Contraindication to imaging with contrast agents
- Any terminal illness with life expectancy < 1 year
- Pregnant women
Primary Results
-
- 300 patients enrolled and randomized
- No patients were lost to follow up
- Median time from stroke onset to hospital arrival ≈ 77min
- Median time from stroke onset to initiation of IV thrombolysis ≈ 132min
- Median time from initiation of IV thrombolysis to arterial puncture ≈ 46min
- Patients receiving thrombolysis without EVT capability ≈ 33%
-
Site of vessel occlusion:
- ICA ≈ 22%
- Basilar ≈ 5%
- MCA M1 ≈ 52%
- MCA M2 ≈ 22%
Critical Findings:
Strengths:
- Study was not sponsored by pharmaceutical company that manufactures tenecteplase (Boehringer Ingelheim)
- Baseline characteristics were well balanced between groups
- Primary and secondary outcomes assessors were blinded to treatment arm
- Outcome assessors were blinded to dose allocation reducing bias
- Primary outcome had to be agreed upon by 2 neuroradiologists and disagreements were resolved by consensus
- Secondary outcomes of freedom from disability and functional independence were pre-specified and adjusted for age and baseline NIHSS score to minimize confounding
- There were no patients lost to follow up and no missing data for the primary or secondary outcomes
- This trial included rural patients (n = 41), largerly recruited via telemedicine, and not represented in the original trial
- 1st substantial RCT to compare 2 doses of tenecteplase for ischemic stroke
Limitations:
- Primary outcome was disease centered (improved perfusion) instead of patient centered (I.e. disability, mortality etc)
- Primary outcome requires interpretation making it somewhat subjective in nature. There was no pre-performed inter-rater reliability assessment
- The authors do not comment on whether patients were recruited consecutively. There may have been selection bias prior to randomization
- The study was underpowered to detect the pre-established minimal clinically important difference (3-5%). The study would have required 2400-6400 patients to achieve 80% power to detect this difference
- CT perfusion was performed but not used to select patients for this trial. Patients with larger core infarcts may have been included in the study, which could potentially dilute results
- Most secondary outcomes were not adjusted for multiple comparisons and therefore these results should be interpreted as exploratory as there is a risk for type I error (rejection of a true null hypothesis = “false positive”)
- Study also not powered to exclude between-group differences in symptomatic ICH and mortality
- Although not statistically significant there was a higher median ischemic core volume and perfusion lesion volume in the patients receiving 0.4mg/kg of tenecteplase compared to those receiving 0.25mg/kg (11mL vs 7mL). Larger ischemic core area and perfusion lesion volumes have the potential to bias results to favor functional neurologic outcomes in the 0.4mg/kg cohort (i.e. more chance of benefit)
Discussion:
- Once again, we see the low frequency of patients who qualify for endovascular care presenting in the 4.5-hour window. The study enrolled 300 patients over 20 months across 28 centers which is less than 1 patient/month/center
- This study would have required an order of magnitude more patients than enrolled to reliably detect the possible clinical importance. Why perform a study that has no hopes in enrolling the number of patients necessary?
- Limited prior research (as noted earlier) questions whether systemic thrombolysis is necessary at all in patients who are eligible for EVT (Phan 2017, Rai 2018). The SKIP study investigated this question and the preliminary findings did not show a benefit to systemic lysis. A complete review of this study when published will be important in guiding practice as well as future research.
- This study included basilar artery occlusions in the group of LVOs eligible for EVT whereas prior studies only included MCA, ICA and ACA vessels.
- Reperfusion prior to endovascular thrombectomy observed with tenecteplase occurred predominantly in patients with MCA occlusions. None of the 66 patients with ICA occlusion achieved the primary outcome (16% of which had partial recanalization potentially improving collateral flow)
- Post hoc analysis demonstrated longer time from systemic thrombolysis to EVT in rural patients and higher substantial reperfusion. This is an interesting finding but needs further data prior to any conclusions being drawn
- When data was pooled with the EXTEND-IA trial, Reperfusion of >50% of the Vascular Territory of the Occluded Vessel was more common with all doses of tenecteplase (20%) vs alteplase (9.9%) aRR 1.90 (95% CI 1.02 – 3.53; p = 0.04). This met pre-specified inferiority and superiority criteria
- There are a number of future trials currently enrolling for the optimal first-line thrombolytic for stroke: TASTE (ACTRN12613000243718), ATTEST2 (NCT02814409), NORTEST2 (NCT03854500)
Authors Conclusions:
“Among patients with large vessel occlusion ischemic stroke, a dose of 0.40mg/kg, compared with 0.25mg/kg, of tenecteplase did not significantly improve cerebral reperfusion prior to endovascular thrombectomy. The findings suggest that the 0.40-mg/kg dose of tenecteplase does not confer an advantage over the 0.25-mg/kg dose in patients with large vessel occlusion ischemic stroke in whom endovascular thrombectomy is planned.”
Our Conclusions: A higher dose of tenecteplase did not result in an improvement of the disease centered primary outcome of improved perfusion. Additionally, there was no difference in patient centered outcomes that were investigated as secondary outcomes. Though not clinically significant, there was a 3-fold increase in intracranial hemorrhage with the higher dose.
Potential to Impact Current Practice: Standard doses of systemic lytics should be continued to be used in patients presenting with LVO acute ischemic strokes who present within established timelines and are eligible for EVT. The bigger question is whether future research will show that systemic thrombolytics are unnecessary in these patients all together.
For More on This Topic Checkout:
- REBEL EM: Endovascular Therapy for Acute Ischemic Stroke
- REBEL EM: Tenecteplase versus Alteplase Before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK)
References:
- Phan K et al. Endovascular thrombectomy alone versus combined with intravenous thrombolysis. World Neurosurg 2017; 108: 850-8. PMID: 28823660
- Rai AT et al. Intravenous thrombolysis before endovascular therapy for large vessel strokes can lead to significantly higher hospital costs with our improving outcomes. J Neurointerv Sure 2018; 10(1): 17-21. PMID: 28062805
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post EXTEND-IA TNK Part II – What Dose of Tenecteplase is the Right Dose? appeared first on REBEL EM - Emergency Medicine Blog.
