
 Background: We have covered tranexamic acid (TXA) on this blog in several posts. Its use has been studied for everything that bleeds from abnormal uterine bleeding to GI hemorrhage and from multisystem trauma to intracranial hemorrhage. While over the past few years it has been touted as the wonderdrug for bleeding, newer research is beginning to challenge that thought (CRASH-3 trial, HALT-IT trial, etc.).
Background: We have covered tranexamic acid (TXA) on this blog in several posts. Its use has been studied for everything that bleeds from abnormal uterine bleeding to GI hemorrhage and from multisystem trauma to intracranial hemorrhage. While over the past few years it has been touted as the wonderdrug for bleeding, newer research is beginning to challenge that thought (CRASH-3 trial, HALT-IT trial, etc.).
The CRASH-2 trial showed that early administration of TXA (within 3 hours) to trauma patients improved all-cause mortality. However, obtaining rapid IV access in low resource, rural, or combat settings can be challenging. Only recently has research been conducted about intramuscular administration of TXA. Actually…we should really say that there has been a resurgence of interest in IM TXA. There were a couple studies published about its pharmacokinetics and pharmacodynamics in the 1970s and 80s, followed by radio silence on the subject.1,2 Curiosity about the drug has picked back up over the past decade as its cost dropped and access to TXA increased exponentially. In fact, finding alternative routes of TXA administration in postpartum hemorrhage is a WHO priority.3
Today, we will review a recent article that explored the pharmacokinetics of intramuscular TXA in bleeding trauma patients.
Paper: Grassin-Delyle et al. Pharmacokinetics of intramuscular tranexamic acid in bleeding trauma patients: a clinical trial. British Journal of Anaesthesia 2020. PMID: 330109274
Clinical Question: In bleeding trauma patients, will intramuscular TXA achieve concentrations sufficient to inhibit fibrinolysis?
What They Did:
Study Design: Prospective, open-label pharmacokinetic study in two London hospital EDs
Population: 30 adult trauma patients who received an IV TXA 1g loading dose, in whom a second TXA dose was indicated
Intervention:
- 0.5g TXA administered into 2 separate sites (at the thigh, gluteal, or deltoid muscles) for a total of 1g
- Serum TXA concentration was measured at various time points
- The IM injection site was inspected each time blood was sampled
Outcomes:
- Baseline data collected on numerous patient demographics and clinical parameters
- TXA concentration was measured using liquid chromatography on blood sampled at the following times (or as soon as possible if patient care precluded blood collection):
- 10 min after IV TXA
- Immediately before IM TXA
- 15 min after IM TXA
- 45 min after IM TXA
- 90 min after IM TXA
- 3 hours after IM TXA
- 6 hours after IM TXA
- 10 hours after IM TXA
- Adverse events recorded for up to 7 days
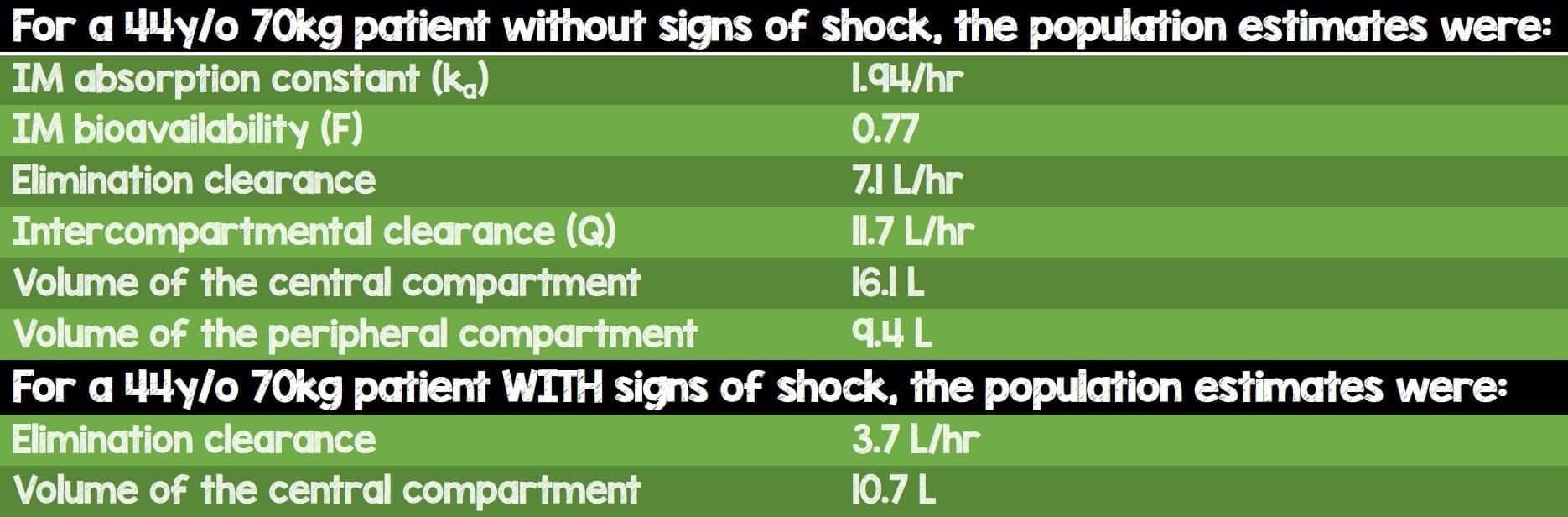
- Pharmacokinetic parameter estimates for a 44y/o 70kg patient
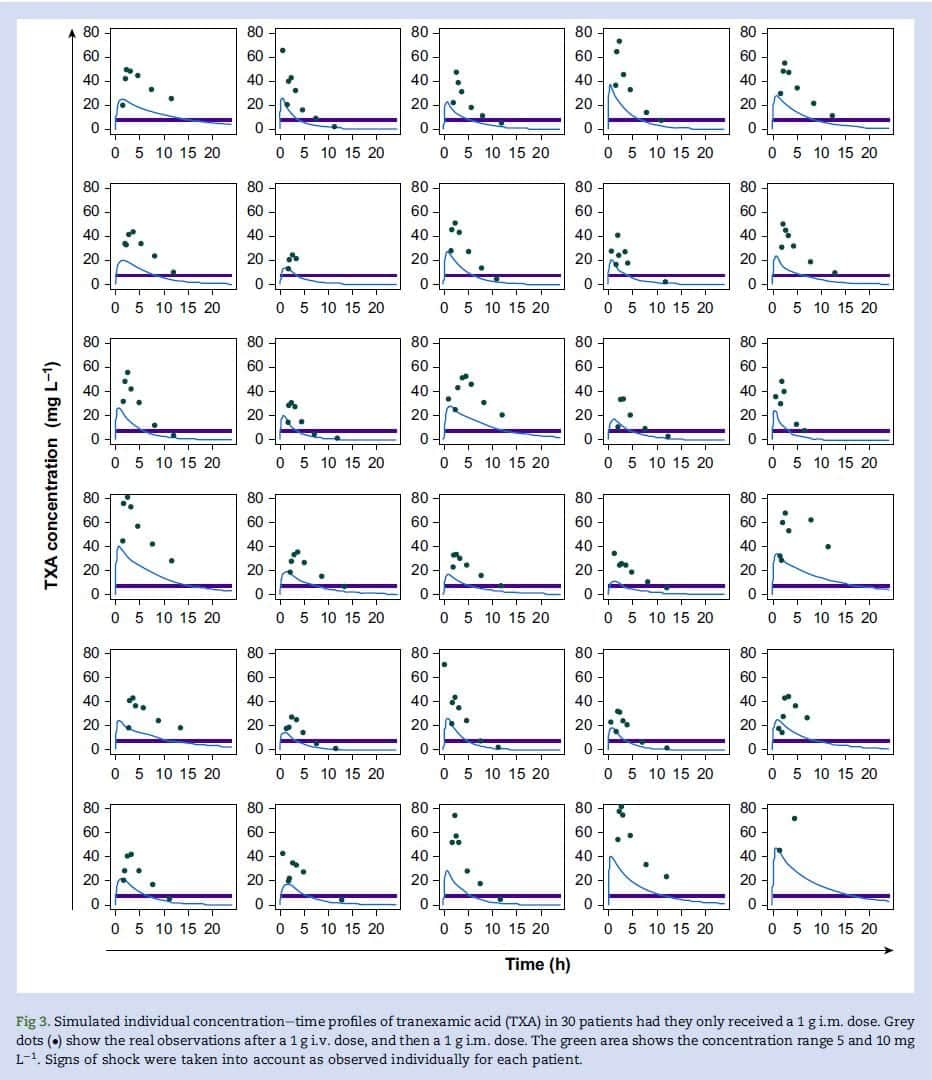
- Simulated concentration-time curves for 1mg IM TXA administration based on data collected from the study
Results:
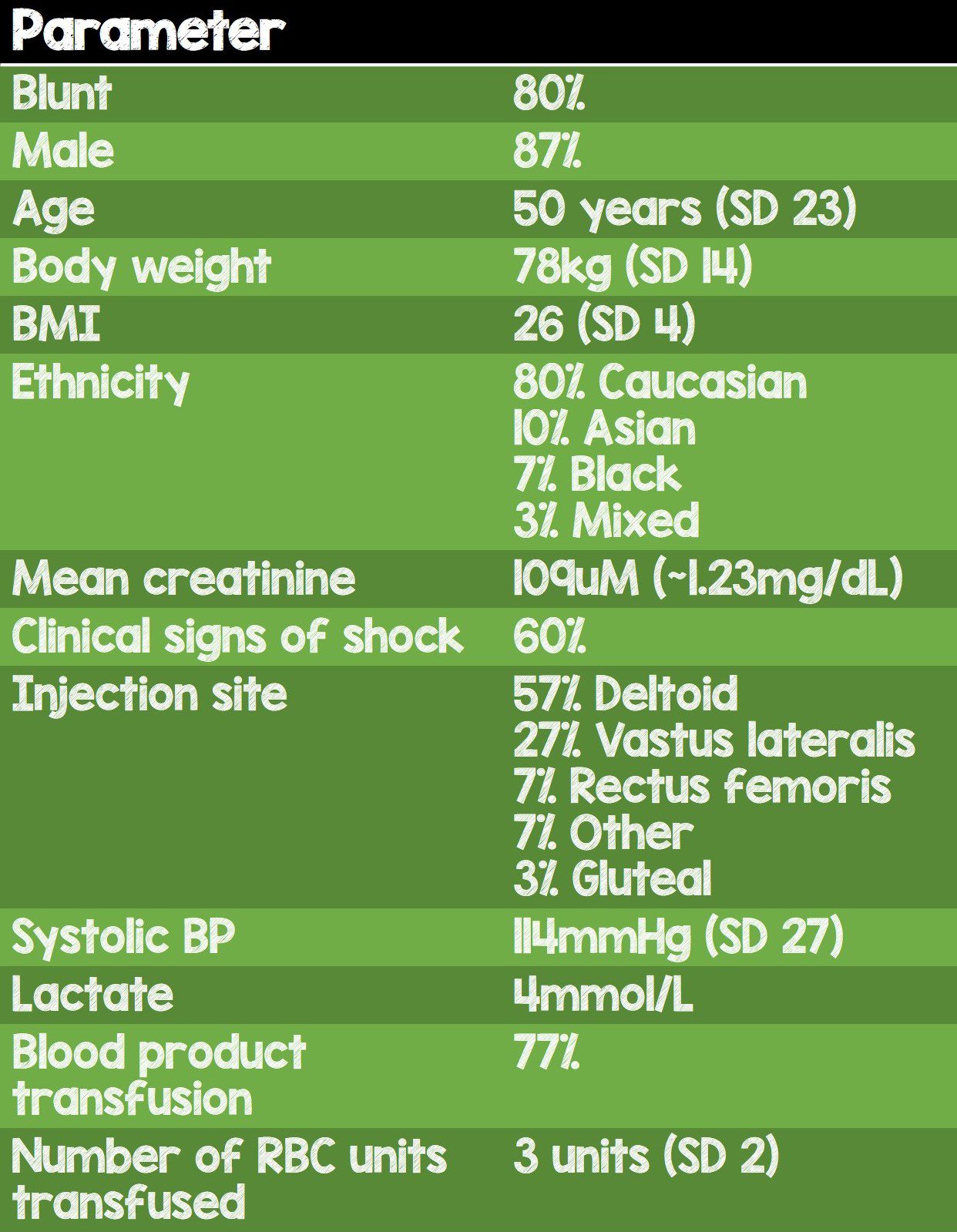
- Total of 30 patients received IM TXA
- 24 patients had blunt injury and 6 had penetrating injury
- Time from injury to IV TXA dose: 1.1hr
- Time between IV and IM TXA doses: 1.8hrs
- The data demonstrated that IM TXA follows a two-compartment open model with first-order absorption and elimination
- After a single TXA dose of 1g IM a concentration of 5mg/L would be achieved in ≈4min and remain above this level for 10h
- After a single TXA dose of 1g IM a concentration of 10mg/L would be achieved in ≈11min and remain above this level for 5.6h
- After a single TXA dose of 0.5g IM a concentration of 5mg/L would be achieved in ≈10min and remain above this level for 5.8h
- After a single TXA dose of 0.5g IM a concentration of 10mg/L would be achieved in ≈20min (in 22 out of 30pts) and remain above this level for 2.8h
- For the pharmacologists in our audience, here are some important parameter estimates
- Using the patient-specific TXA pharmacokinetics, they created simulated concentration-time profiles for 1g IM TXA to determine the duration of concentration over 5mg/L and 10mg/L
- Injection site, BMI, sex, ethnicity, GFR, type of injury, blood lactate levels, and signs of shock had no apparent impact on absorption and pharmacokinetics.
Strengths:
- First and foremost, the study was registered under a pretty cool short-form name: TraumaINTACT
- 1st study to examine serum concentrations and pharmacokinetics of IM TXA in bleeding trauma patients
- Prospective study
- Collected 8 data points per patient, up to 12 hours from the IM injection
- Fairly sick population with clinical signs of shock in 60% of study participants, average lactate of 4mmol/L; 77% of participants received blood products with an average of 3 RBC units transfused.
- They looked into the effect of patient parameters on absorption or pharmacokinetics
- Investigators performed regular inspection of the IM injection site looking for adverse reactions
- DATA WAS MADE FREELY AVAILABLE ON THE FREEBIRD BANK OF INJURY AND EMERGENCY RESEARCH DATA WEBSITE
- No pharma involvement in the study
- Collected data on treatments that may have influenced TXA concentrations (i.e. blood products and IV fluids)
Limitations:
- The authors do not specify whether this was a consecutive sample or convenience sample
- Small study involving only 30 patients
- Because patient care was the priority (as it should be), they were unable to obtain a blood sample at 10 minutes after the IV injection in all patients; however, all participants had a blood sample obtained prior to IM administration.
- The study only included 4 females; while trauma disproportionately affects males more than females, this study does not quite reflect the appropriate degree to which that occurs. Because males and females have differences in drug absorption, metabolism, and excretion,5 this may limit generalizability of the data.
Discussion:
- This is an important trial that shows IM TXA is feasible in trauma patients. It could be given by trained first responders, police officers, and primary care nurses with important reductions in time to treatment even without IV access, particularly in low- and middle-income countries with less developed prehospital systems of care
- We know that TXA works for multisystem trauma if given within 3 hours after the injury. Intravenous administration is currently the standard route, but this study takes the first steps in the exploration of intramuscular administration. I commend the authors for exploring this topic and am excited by the potential impact this study has on global health.
- They appropriately note in the introduction that hemorrhagic shock from trauma decreases blood flow to skin and skeletal muscle, potentially impacting the absorption from the IM depot. However the parameter estimates obtained in this trial are consistent with those from healthy volunteers
- The same authors published a meta-analysis of TXA pharmacokinetics last year, comparing IM vs IV vs oral routes of administration.6 Only 6 patients in the study received TXA via the IM route.
- The concentration of TXA required for antifibrinolysis was determined by this study. The systematic review included 20 in vitro studies and 1 in vivo study and concluded that concentrations of 10-15mg/L is sufficient to inhibit fibrinolysis.
- In this small study, there were no serious adverse events noted except for transient erythema at the injection site and one patient who had pyrexia 2 days after the injection. It is unlikely that the pyrexia was caused by TXA given that it occurred 2 days after administration of the medication.
- Injection site, BMI, sex, ethnicity, GFR, type of injury, blood lactate levels, and signs of shock had no apparent impact on absorption and pharmacokinetics.
- This study is hypothesis-generating only and cannot be used to definitively recommend IM administration of TXA. However, it does convincingly show that IM TXA can achieve concentrations well above the concentrations needed for fibrinolysis within minutes.
- Until we have large multicenter randomized controlled trials, we cannot change our practice or recommendations about IM administration of TXA for trauma.
- In a cliff-hanger at the end of the article, the authors mention that a similar study is underway in postpartum hemorrhage patients.
Author Conclusions:
“Intramuscular TXA is well tolerated with only mild and transient injection site reactions. Intramuscular TXA is rapidly absorbed, reaching therapeutic concentrations within 15 min. Blood lactate and signs of shock had no apparent impact on the rate of absorption. Our results have major implications for trauma care, particularly in low- and middle-income countries where i.m. TXA could expand access to treatment.”
Clinical Take Home Point:
IM TXA appears to be rapidly absorbed and reaches therapeutic concentrations quickly. While this paper does not change our current clinical practice here in the US, it does open doors for further research and potentially impacts the care of trauma patients in healthcare systems of low- and middle-income countries.
References:
- Sano M et al. Absorption and Excretion of Tranexamic Acid following Intravenous, Intramuscular and Oral Administrations in Healthy Volunteers. Japanese Journal of Clinical Pharmacology and Therapeutics 1976. [Link is HERE]
- Puigdellívol E et al. Pharmacokinetics and absolute bioavailability of intramuscular tranexamic acid in man. International Journal of Clinical Pharmacology and Therapeutics 1985. PMID: 4018929
- World Health Organization. Updated WHO recommendation on tranexamic acid for the treatment of postpartum haemorrhage 2017. [Link is HERE]. [Accessed 29 Oct 2020]
- Grassin-Delyle et al. Pharmacokinetics of intramuscular tranexamic acid in bleeding trauma patients: a clinical trial. British Journal of Anaesthesia 2020. PMID: 33010927
- Whitley H et al. Sex-based differences in drug activity. Am Fam Physician 2009. PMID: 19961138
- Grassin-Delyle S et al. Tranexamic acid through intravenous, intramuscular and oral routes: an individual participant data meta-analysis of pharmacokinetic studies in healthy volunteers. Fundam Clin Pharmacol 2019. PMID: 31013357
For More Thoughts on This Topic Check Out:
- The Resus Room: Papers of November 2020
- Emlyn’s Blog: JC: Can we give tranexamic acid (TXA) via the IM route?
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post IM Administration of Tranexamic Acid appeared first on REBEL EM - Emergency Medicine Blog.
