
 The opioid epidemic has ignited a revolution in ED pain management over the last 5+ years. As such, opportunities for research have become more abundant and have driven ED pain management forward. Regional anesthesia, lidocaine infusions, intranasal ketamine, etc., are now a regular part of ED treatment algorithms.
The opioid epidemic has ignited a revolution in ED pain management over the last 5+ years. As such, opportunities for research have become more abundant and have driven ED pain management forward. Regional anesthesia, lidocaine infusions, intranasal ketamine, etc., are now a regular part of ED treatment algorithms.
Multiple studies have investigated the analgesic effects of ketorolac for various conditions. However, this paper is the first to investigate the efficacy of intramuscular ketorolac based on dose for acute musculoskeletal pain.
Paper: Turner NJ et al. Comparing Two Doses of Intramuscular Ketorolac for Treatment of Acute Musculoskeletal Pain in a Military Emergency Department. Am J Emerg Med. 2021. [Link is HERE]
Clinical Question: Is 15 mg of IM ketorolac noninferior to 60 mg of IM ketorolac for patients with mild acute MSK pain?
What They Did:
-
Single-blind, randomized controlled noninferiority trial
-
-
- Patients were blinded to the IM dose of ketorolac administered
-
-
- Selected a convenience sample of patients presenting to a Defense Health Agency facility serving Department of Defence and Veterans Affairs
- Patients randomized to receive either 15 mg or 60 mg of IM ketorolac
- Participants marked their pain on a 100 mm visual analog scale (VAS) immediately before ketorolac administration and again after 30 min and 60 min.
- Noninferiority margin set at 13mm.
- Therefore, a difference in 13 mm between the two doses would have shown inferiority of the 15 mg dose.
Inclusion:
- Adults aged 18 – 55 years of age
- Reported with acute MSK pain less than 30 days
- Reported a pain intensity of 20 or greater on a standard VAS
- Triaged as emergency severity index (ESI) category of four or five
- EM physician agreed that IM ketorolac was appropriate
Exclusion:
- Patient weight less than 50 kg (110 lbs)
- Pregnant or breastfeeding
- NSAID allergy or hypersensitivity
- Any analgesic use within 12 h
- History of renal disease
- Bleeding diathesis
Primary Outcome:
- Change in VAS pain score 60-min after IM ketorolac administration
Secondary Outcomes:
- Change in VAS pain score 30-min after IM ketorolac administration
- Incidence of reported adverse effects with the administration of ketorolac
Results:
-
175 patients screened
-
-
- 65 excluded
- 110 recruited to participate
- 27% female
- Mean age of 30.9
- The most common reason for exclusion: analgesics taken within 12h of presentation
-
-
-
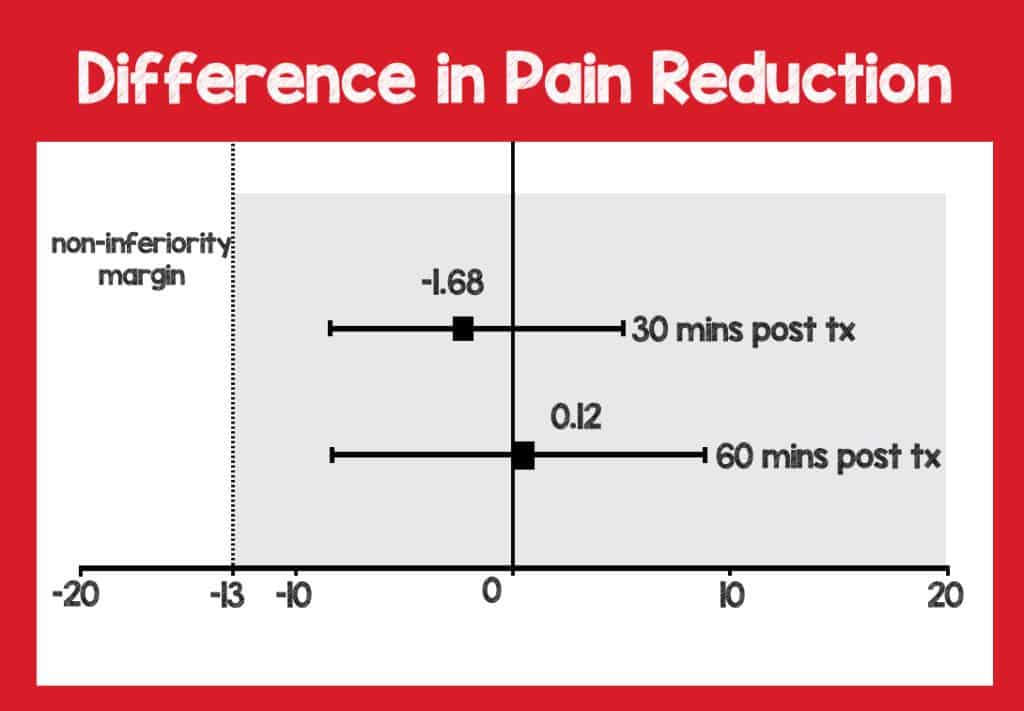
Primary Outcome: Change in VAS score at 60-min: 15 mg found to be NONINFERIOR to 60 mg
-
-
- The mean difference between groups was 0.1 mm on VAS (95% CI -8.5 – 8.7; p = .98)
- The lower dose achieved the noninferiority margin (set at 13 mm on VAS)
-
-
-
Secondary Outcome: Change in VAS score at 30-min: 15 mg found to be NONINFERIOR to 60 mg
-
-
- The mean difference between groups was -1.7 mm on VAS (95% CI -8.5 – 5.1; p = .63)
-
-
- No major adverse effects were reported at 60 min
-
Minor effects were more frequent in the 60 mg group
-
-
- 16.4% vs 1.8%
- Burning at the injection site was the most common adverse effect reported
-
-
-
Post Hoc Analysis
-
-
- Patients with back or neck pain reported statistically significant pain reduction at 30 min regardless of the dose of medication
-
-
Strengths:
- Patients were blinded to medication limiting placebo effect
- Blinded randomization protocol
- No patients required rescue medication
- Adds to the growing body of literature to support lower doses of ketorolac
Limitations:
-
Single-blind study
-
-
- Only patients were blinded to the dose of ketorolac administered
- Study investigators and nurses were aware of medication being administered
- Randomization occurred before collection, then data sheets were populated and placed in envelopes.
- Envelope systems can be hacked and this has happened in past.
-
-
- Study participants are a convenience sample which causes selection bias
-
A small, single-center study performed in a military hospital whose patient population likely differs significantly from an individual physician in an inner-city academic center or small community hospital limiting generalizability
-
-
- 82% of patients were active military
-
-
-
It is unclear if patients in the different arms had similar treatment outside of the intervention (ketorolac).
-
-
- The article does not state if patients were concomitantly given other analgesics or if there were differences in other analgesics administered.
- They do state that no rescue analgesia was required by either group.
-
-
- Did not perform an intention-to-treat analysis only performed per-protocol analysis which does not reflect real-world conditions
-
Excluded patients with ESI of 1-3
-
-
- In some institutions including my own, a pain of ≥7 on a numeric scale, regardless of cause, would be considered an ESI of 3
-
-
- Adverse effects examined up to 60 min some adverse effects may take longer to manifest
- Serious adverse effects such as gastrointestinal bleeding and renal injury were not investigated
Discussion:
-
In this study, patients were blinded to medication administration, but study investigators were aware of medication dosage.
-
-
- Investigators used randomization software and blind envelopes to minimize bias.
- But single blinding can still result in bias.
- Investigators who knew the dose could influence the assessment of pain by theoretically asking questions in a loaded manner
-
-
- Additionally, study participants were selected as a convenience sample. We know nothing of patients who were eligible for enrollment but not randomized, further adding to selection bias.
- 82% of patients were active military which is likely very different from most ED patient populations potentially limiting generalizability and extrapolation of data.
-
The investigators performed a per-protocol analysis but did not perform an intention-to-treat analysis.
-
-
- ITT analysis represents patients randomized to a particular therapy, whether they were adherent or not.
- ITT is more reflective of real-world experience.
- Per-protocol analysis represents patients randomized to a particular therapy and who were adherent to the study protocol
-
-
- The study protocol included patients with mild pain (VAS ≥20).
- The investigators report that only 15% of patients had a VAS score of < 50 and average pretreatment VAS scores of 68. Why investigate parenteral pain management in a population in which many physicians would prescribe ibuprofen or another PO NSAID?
- If the patient tolerates PO, there is no reason to give an IM injection. Oral NSAIDs have been shown to be equally effective though they may be slightly more delayed in onset. IM ketorolac is known to be painful
- Though IM analgesics are still routinely given If parenteral medications are required, IV analgesics may be a better option. Why cause pain to treat pain, especially mild pain?
- The investigators performed post hoc analysis to determine if pain reduction differed by age, gender, body weight, duration of pain, or location of pain. It may have been helpful to analyze based on VAS scores and investigate if there was a difference in patients with more severe pain vs. less severe pain.
- Pioneers in ED pain management such as Dr. Sergey Motov have moved the needle advocating for lower doses of ketorolac. Many physicians have thus subsequently changed their practice [Link is HERE].
- It bears mentioning that physicians often have a pragmatic sensibility when administering analgesics characterized by the thought that you can always give more pain meds if needed. Therefore it may be prudent to start with lower doses of medications.
Author’s Conclusion:
“A 15 mg IM dose of ketorolac was found to be non-inferior to a 60 mg dose for short-term pain relief of acute MSK pain in adults presenting to an ED. Subjective adverse effects, while not numerous, were more often reported with the 60 mg dose. Discontinuing the practice of ordering 60 mg IM doses of ketorolac in place of a lower dose for acute MSK pain should be considered.”
Our Conclusion: Though not without limitations, this paper adds to the growing body of evidence to support the use of lower doses of ketorolac in various conditions.
Clinical Bottom Line:
If you have not already done so, lower the dose of ketorolac routinely administered. Make it easy to prescribe lower doses of ketorolac and hard to prescribe higher doses. Save ketorolac with a lower dose preset in your EMR favorites, and delete the higher doses if needed.
References:
- Motov S et al. Comparison of Intravenous Ketorolac at Three Single-Dose Regimens for Treating Acute Pain in the Emergency Department: A Randomized Controlled Trial. Ann Emerg Med. 2017;70(2):177-184. [Link is HERE]
- Guyatt G, Rennie D, Meade M, Cook D. Users’ Guides To The Medical Literature. 3rd ed. McGraw-Hill Education; 2015. [Link is here]
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Ketorolac for Acute MSK Pain: 15mg IM vs 60mg IM appeared first on REBEL EM - Emergency Medicine Blog.
