
 Background: Since the introduction of SARS-CoV-2 to the world in December 2019, there have been no medications approved or proven effective for the treatment of this pandemic. Lopinavir is an HIV protease inhibitor that is combined with Ritonavir to increase its half-life. This combination of medications has also been studied in severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) and showed promising results. This makes it a research target for COVID-19. Many of us are awaiting a treatment that works against SARS-CoV-2 and badly want/need a treatment that is safe and effective. In this publication the authors evaluated the efficacy and safety of oral lopinavir-ritonavir for SARS-CoV-2 infection in adult patients hospitalized with severe COVID-19.
Background: Since the introduction of SARS-CoV-2 to the world in December 2019, there have been no medications approved or proven effective for the treatment of this pandemic. Lopinavir is an HIV protease inhibitor that is combined with Ritonavir to increase its half-life. This combination of medications has also been studied in severe acute respiratory syndrome (SARS) and Middle East respiratory syndrome (MERS) and showed promising results. This makes it a research target for COVID-19. Many of us are awaiting a treatment that works against SARS-CoV-2 and badly want/need a treatment that is safe and effective. In this publication the authors evaluated the efficacy and safety of oral lopinavir-ritonavir for SARS-CoV-2 infection in adult patients hospitalized with severe COVID-19.
Paper: Cao B et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. NEJM 2020. [Epub Ahead of Print]
Clinical Question: Does the combination of lopinavir-ritonavir + standard care reduce the time to clinical improvement in patients with severe COVID-19 infection compared to standard care alone?
What They Did:
- Individually randomized, controlled, open-label trial involving hospitalized adult patients with confirmed SARS-CoV-2 infection, O2 saturation ≤94% while breathing ambient air or a P/F ratio of ≤300mmHg
- Study took place in Wuhan, China
- Patients were randomly assigned to:
- Intervention: Lopinavir-ritonavir 400mg/100mg BID for 14d + standard care
- Control: Standard care alone
- Standard care included supplemental oxygen, noninvasive and invasive ventilation, antibiotic agents, vasopressor support, renal-replacement therapy, and extracorporeal membrane oxygenation (ECMO)
- To balance the distribution of oxygen support between groups as an indicator of severity of respiratory failure, randomization was on the basis of respiratory support methods at the time of enrollment:
- No oxygen support
- Oxygen support with nasal cannula or mask
- Oxygen support with HFNC
- Oxygen support with NIV
- Oxygen support with invasive ventilation including ECMO
- Serial oropharyngeal swab samples were obtained on day 1 (before intervention was administered) and on days 5, 10, 14, 21, and 28 until discharge or death had occurred using RT-PCR
- Sampling did not stop when a swab at a given point was negative.
Outcomes:
- Primary: Time to clinical improvement (defined as time from randomization to either an improvement of two points on a seven-category ordinal scale or discharge from the hospital, whichever came first)
- 7-point ordinal scale
- 1 = Not hospitalized with resumption of normal activities
- 2 = Not hospitalized but unable to resume normal activities
- 3 = Hospitalized, not requiring supplemental oxygen
- 4 = Hospitalized, requiring supplemental oxygen
- 5 = Hospitalized, requiring nasal high-flow oxygen therapy, NIV, or both
- 6 = Hospitalized, requiring ECMO, invasive mechanical ventilation, or both
- 7 = death
-
Secondary:
- Clinical status as assessed with the seven-category ordinal scale on days 7 and 14
- 28d mortality
- Duration of mechanical ventilation
- Duration of hospitalization in survivors
- Time (in days) from treatment initiation to death
- Proportion with viral RNA detection over time
- Viral RNA titer area under the curve (AUC)
-
Safety:
- Adverse events during treatment
- Serious adverse events (Respiratory failure or ARDS, AKI, secondary infection, shock, severe anemia, acute gastritis, GIB, pneumothorax, unconsciousness, DIC, sepsis, acute heart failure)
- GI adverse events (Nausea, vomiting, and diarrhea)
- Premature discontinuation of treatment
Inclusion:
- Positive reverse transcriptase polymerase chain reaction (RT-PCR) assay
- Adult patients ≥18 years of age
- Pneumonia confirmed by chest imaging
- O2 saturation ≤94% on room air
- P/F ration ≤300mmHg
Exclusion:
- Physician decision that involvement in trial was not in patient’s best interest
- Presence of any condition that would not allow protocol to be followed safely
- Known allergy or hypersensitivity to lopinavir-ritonavir
- Known severe liver disease (i.e. cirrhosis, with ALT >5x the upper limit of the normal range)
- Use of medications that are contraindicated with lopinavir-ritonavir and could not be replaced or stopped during the trial period
- Pregnancy
- Breast-feeding
- Known HIV infection (Due to concerns about development of resistance to lopinavir-ritonavir if used without combing with other antiretrovirals)
- Unable to swallow receive medication through a NG tube
Results:
- 199 patients underwent randomization
- Median age was 58 years (Range 49 to 68)
- Median interval time between symptom onset and randomization was 13d (Range 11 to 16d)
-
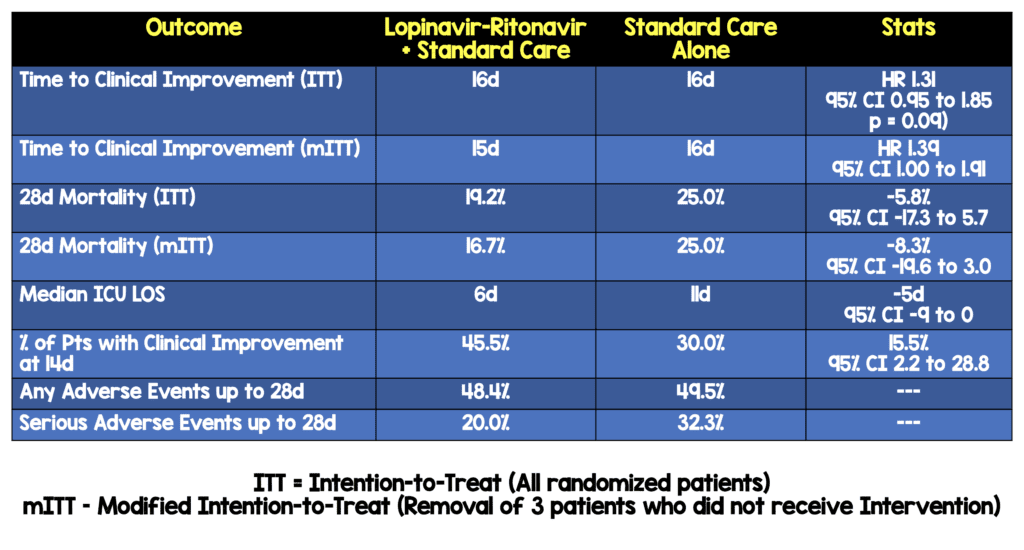
Intervention treatment within 12d after symptom onset was associated with shorter time to clinical improvement (HR 1.25; 95% CI, 1.77 to 2.05)
- This was the statement on March 18th, 2020…however on March 26th, 2020…
- Intervention treatment within 12d after symptom onset WAS NOT associated with shorter time to clinical improvement (HR 1.25; 95% CI 0.77 to 2.05)

- Intervention treatment >12d after symptom onset was not associated with shorter time to clinical improvement (HR 1.30; 95% CI 0.84 to 1.99)
- No significant differences in duration of O2 therapy, duration of hospitalization, and time from randomization to death
- There was no difference between groups in viral RNA loads over time
- Intervention was stopped early in 13 patients (13.8%) because of adverse events
- Percentages of patients with detectable virus at various time points were similar
Strengths:
- First published study asking a clinically important question regarding COVID19 treatment
- To minimize allocation bias, the authors performed allocation concealment until randomization was finished
- Nasopharyngeal samples were obtained for all 199 patients who were still alive at every time point
- Used an intention-to-treat analysis which includes all patients that are randomized regardless of protocol violations which is more like clinical practice
- Time to clinical improvement was assessed after all patients had reached day 28 with failure to reach clinical improvement or death before day 28
- Performed a modified intention-to-treat analysis that excluded three early deaths was also performed
- No between group differences in demographics, baseline laboratory tests, distribution of ordinal scale scores, or NEWS2 scores at enrollment
Limitations:
- 5 patients (5%) in the intervention group did not receive any doses of intervention (3 of them died in 24hours) and were included in the intention-to-treat analysis which may bias the results to favor standardized care
- The statistical analysis did not have a provision for correcting of multiplicity of tests for secondary or other outcomes, therefore the widths of the confidence intervals were not adjusted for this. Because of this confidence intervals should not be used to infer definitive treatment effects
- Systemic glucocorticoids were administered in 33.0% of patients in the lopinavir-ritonavir group and in 35% of those in the standard-care group. It is hypothesized that steroids can prolong viral replication which could impact the results of this study
- The control group appeared to be clinically sicker than the intervention group with more vasopressors (27.0% vs 17.2%), more NIV (19.0% vs 10.1%), and more RRT (6.0% vs 3.0%). This could bias the results to favor the intervention
- Samples for viral RNA were only taken intermittently and more frequent sampling may have provided more information on viral load kinetics in the two groups
- Throat swab samples were used to test for viral loads which may be why we see no difference in change in viral loads. Throat swabs have been shown to have lower viral loads than nasopharyngeal samples.
- Unblinded trial, which makes it possible that the knowledge of the treatment assignment may have influenced clinical decision making which could affect the primary outcome
- The lopinavir-ritonavir group had slightly higher throat viral loads which could raise the issue of more viral replication
- Use of glucocorticoids in 1/3rd of patients, which is known to increase viral shedding
Discussion:
- An 8 day difference is a rather long time difference for clinical improvement. As an aside, oseltamivir, which is used for influenza only has a half day or full day improvement in symptoms. It may be that this trial was set up for failure by looking for an 8 day difference in clinical improvement
- Due to the emergency nature of the trial, placebos of lopinavir-ritonavir were not prepared
- Planned enrollment was for 160 patients, however after assessment it was decided the trial was underpowered and a decision was made to continue enrollment by the investigators, however another potential medication, remdesivir became available for clinical trials, therefor enrollment was suspended after 199 patients were recruited for randomization
- A post hoc analysis showed that if given earlier (<12d), lopinavir-ritonavir did reduce mortality overall. The question of whether earlier lopinavir-ritonavir treatment in COVID-19 could have clinical benefit is a hypothetical one at this time
- Interestingly, another finding that is worth mentioning, is patients receiving lopinavir-ritonavir had fewer cases of AKI and secondary infections
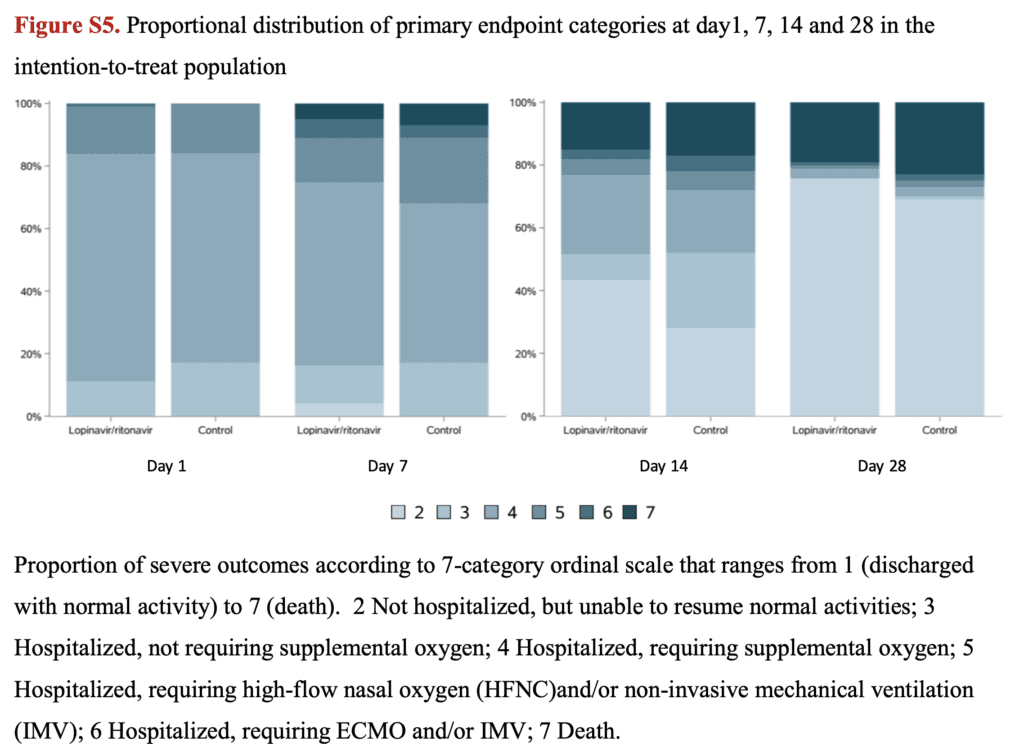
- Buried in the supplement are the proportional distribution of patients on the 7-category ordinal scale. It appears that the severity of illness was greater in the patients treated only with standard care over a 28d period
- The concentration of lopinavir-ritonavir needed to work against SARS-C0V-2 is thought to be pretty high but were fairly low in this study. This would need to be balanced with the potential side effects that would be increased for higher doses or longer regimens that patients may not tolerate.
Author Conclusion: “In hospitalized adult patients with severe COVID-19, no benefit was observed with lopinavir-ritonavir treatment beyond standard care. Future trials in patients with severe illness may help to confirm or exclude the possibility of a treatment benefit.”
Clinical Take Home Point: This was a very sick population with a 25% mortality rate in the control group and it could be that the patients recruited were late in the infection process therefore already having significant lung damage that was irreversible regardless of treatment. Despite the overall negative bottom line of this trial, which may be due to a rather large time difference in improvement of clinical symptoms (8d), there are some very promising results that will require further study including 28 mortality, median ICU length of stay, and percentage of patients with clinical improvement at 14d.
References:
- Cao B et al. A Trial of Lopinavir-Ritonavir in Adults Hospitalized with Severe COVID-19. NEJM 2020. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- PulmCrit: Is Lopinavir/Ritonavir Down and Out?
- The Bottom Line: LOTUS China
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post Lopinavir-Ritonavir in Hospitalized Patients with Severe COVID-19 appeared first on REBEL EM - Emergency Medicine Blog.


