
 Background Information:
Background Information:
Epistaxis management in the emergency department has historically included topical vasoconstrictors, local anesthetics such as oxymetazoline and phenylephrine and topical fibrinolytic agents such as Tranexamic Acid (TXA). When these topical agents fail, packing, chemical cauterization or surgical ligation is often required. Nasal packing is painful and typically requires a subsequent visit or close follow-up for removal. Some limited research has suggested use of TXA improves bleeding control with decreased rebleeding rates compared to some of the other treatment options used.1 The recent NoPAC trial challenged the way providers in the emergency department utilize TXA stating that its effects are no better than placebo at managing uncontrolled epistaxis.2 The authors of the current systematic review and meta-analysis sought to include data from all of these studies to evaluate the effectiveness and potential benefits of topical TXA.
Paper: Janapala RN, et al. Efficacy of Topical Tranexamic Acid in Epistaxis: A Systematic Review and Meta-Analysis. Am J Emerg Med. Nov 2021. PMID: 34763235
Clinical Question:
- Is topical TXA compared to other affordable alternative methods more effective in bleeding cessation of epistaxis?
What They Did:
- Performed a PubMed and Scopus database search from inception to April 2021 with terms related to tranexamic acid and epistaxis. The search was performed in compliance with the 2020 Preferred Reporting Items for Systematic Review and Meta-Analysis (PRISMA)
- To evaluate risk of bias for randomized studies the authors used the Cochrane Risk of Bias Tool. To assess the quality of observational studies, they used the Newcastle-Ottawa Scale
- A cumulative meta-analysis was performed when the earliest study became available and then repeated when a second study was available. It was then repeated when a third study became available and so on.
Inclusion Criteria:
- Randomized trials and observational (prospective or retrospective) studies were included if they:
- Were published in English
- Involved adult patients > 18 years of age
Exclusion Criteria:
- Non-full-text studies
- Conference abstracts
- Non-English publications
- Studies involving epistaxis caused by vascular pathology, surgery, etc
Outcomes:
Primary
- Prevalence of bleeding cessation after treatment with TXA at first re-assessment by the study others
Secondary
- Recurrence of bleeding between 24 and 72 hours
- Recurrence of bleeding 7-8 days after treatment
Results:
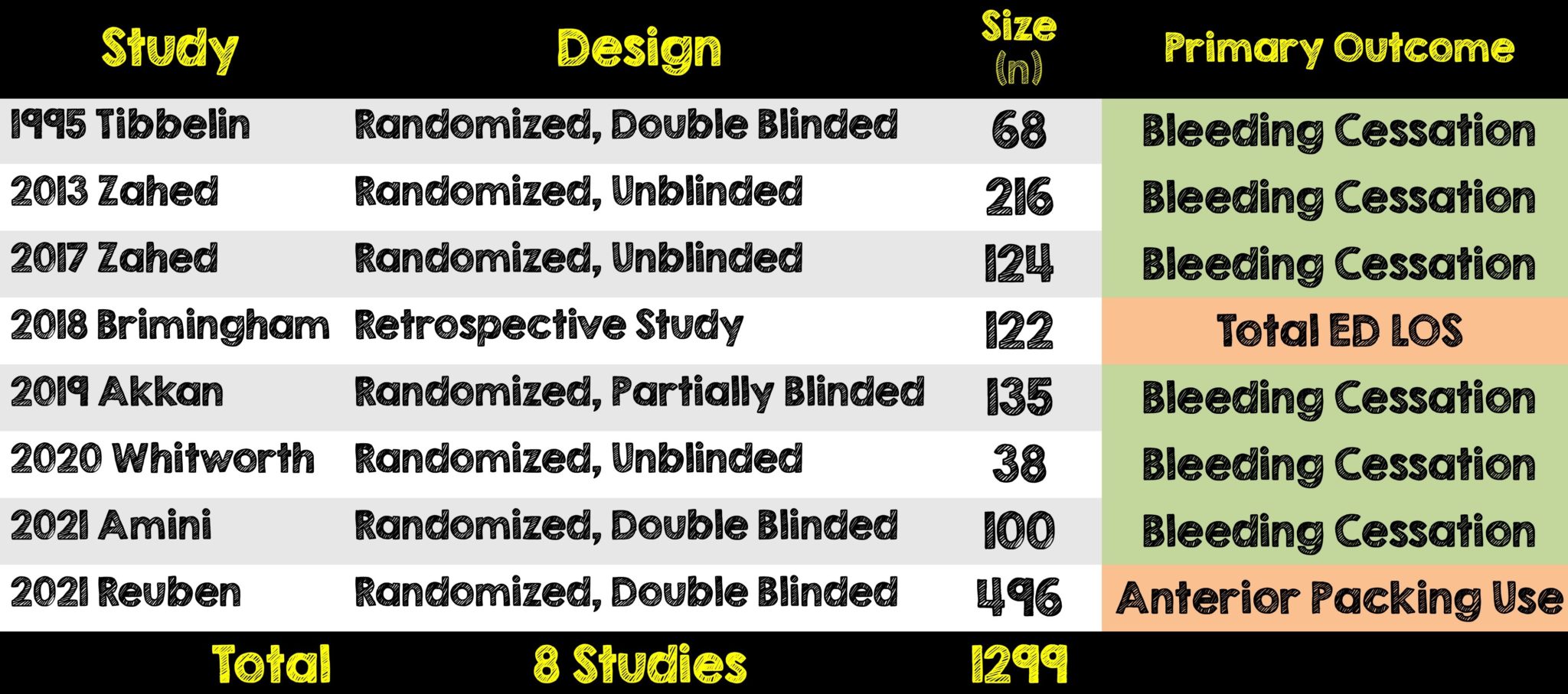
- Of the 2375 eligible studies, 225 were reviewed and 8 were included in the meta-analysis
- Seven of which were randomized trials and one was a retrospective study
- A total of 1299 patients were included
- 596 (46%) received TXA
- 703 (54%) received control treatment of placebo, epinephrine plus lidocaine or oxymetazoline or phenylephrine plus lidocaine
Critical Results:

- According to the Cochrane Risk Bias tool, the overall risk of bias was low for two randomized control trials, while the four other randomized control trials showed some concern for bias (see Figure 1).
- Using Orwin’s Fail-safe N study, the authors calculated that 14 more studies favoring other vasoconstrictors would be needed to make TXA not statistically significant for stopping epistaxis compared to other vasoconstrictors
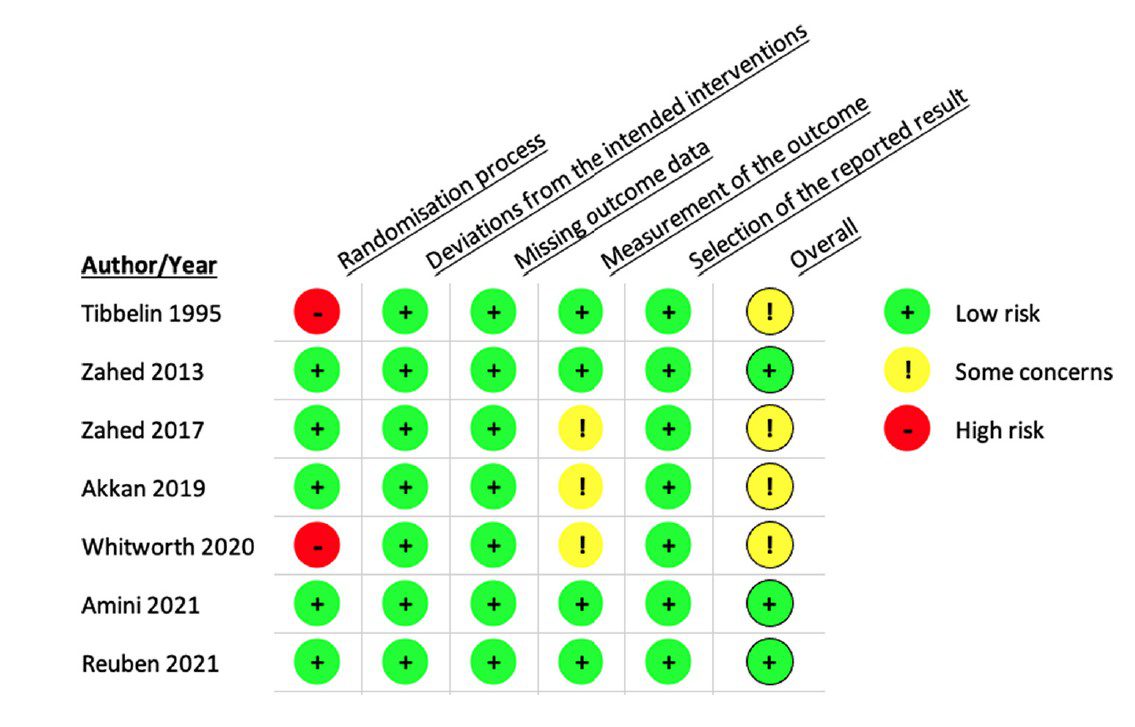
Figure 1: Risk of Bias for Randomized Controlled Trials (Image from original paper)
Strengths:
- Attempts to answer a clinically relevant and now controversial question regarding the use of TXA in epistaxis
- Utilized a clearly outlined and practical primary outcome of bleeding cessation with TXA at first reassessment
- Excluded epistaxis caused by vascular pathology and surgery
- Used tools to evaluate for the risk of bias in randomized studies and scales to assess the quality of observational studies
- Had a test to predict the number of future or missing studies needed that might change the effect size of the primary outcome and reported this number
- Had two additional tests to assess for publication bias and they both showed a lower likelihood that publication bias actually existed
- Large number of patients were included in the total cumulative meta-analysis
Limitations:
- Strict selection criteria resulted in a small number of studies included and therefore overall power to show statistically significant differences in subgroup analysis may be affected
- Majority of the randomized trials included were not double-blinded
- Half of included studies were considered to have concerns for bias and this carries over into the meta-analysis
- Excluded the largest randomized double-blind trial from bleeding cessation meta-analysis due to them not reporting it
- Most studies did not report patient characteristics in details (ie. Hypertension history, anticoagulation/antiplatelet use, etc) and thus authors did not perform further statistical analysis for association of these factors
Discussion:
- The number of studies in this meta-analysis is noteworthy. This is not the first meta-analysis to evaluate specifically for the cessation of bleeding following topical application of TXA. The other one by Gottlieb et al did not reach statistical significance with the TXA group likely because of insufficient power as their study only included a total of three studies3
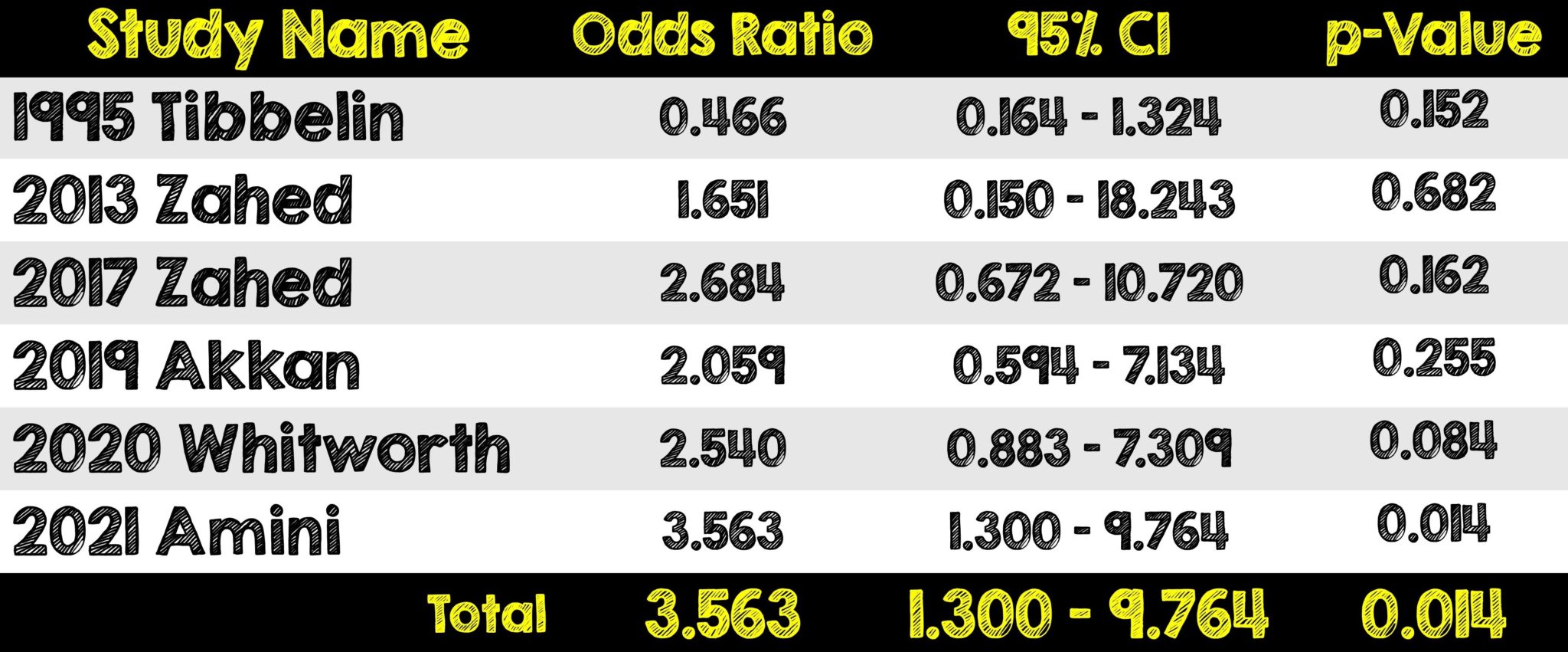
- The efficacy of TXA on bleeding cessation when compared to other vasoconstrictors is striking. It was 7.8x more likely to achieve bleeding control on first assessment and lowered the odds of recurrence of bleeding at days 1 and 3.
- The authors omitted the randomized control trial with the largest number of patients because the NoPAC trial was notably different then the other 7 trials reviewed.
- The NoPAC trial (which we covered on REBEL before) found TXA to be no more effective than placebo AFTER conservative measures like vasoconstrictors and direct pressure were applied first.
Author’s Conclusions:
- Topical TXA is associated with better bleeding cessation rates after treatment compared to the standard practices.
Our Conclusion:
- This meta-analysis highlights the importance of the additional studies needed to specifically compare TXA’s effect on the cessation of bleeding time to alternative vasoconstrictors once basic measures for bleeding control have been attempted and failed. The use of topical TXA in the management of epistaxis may lead to faster cessation of bleeding time when compared to other vasoconstrictors, however given the findings of the recent NoPAC trial, it’s use should be left up to the discretion of the emergency physician.
Clinical Bottom Line:
- This meta-analysis highlights the importance of a head-to-head comparison of first line agents in the treatment of epistaxis. Knowing the efficacy of topical TXA compared to other first line vasoconstrictors from this study, it’s not an unreasonable approach to use it as a first line agent. The use of TXA in epistaxis should be left to the discretion of the emergency physician. Although, the NoPAC trial showed no difference in the use of topical TXA, that study was different in that direct pressure plus phenylephrine was used and only after failure were patients randomized to TXA vs placebo. Maybe a better strategy would be direct pressure PLUS TXA as a 1st line approach to epistaxis as it appears from this study that it does a better job in bleeding cessation when compared to other vasoconstrictors.
REFERENCES:
- Janapala RN, et al. Efficacy of topical tranexamic acid in epistaxis: A systematic review and meta-analysis. Am J Emerg Med. Nov 2021 Epub ahead of print. PMID: 34763235
- Reuben A, et al. The Use of Tranexamic Acid to Reduce the Need for Nasal Packing in Epistaxis (NoPAC): Randomized Controlled Trial. Ann Emerg Med. Jun 2021. PMID: 33612282
- Gottlieb M, et al. Topical Tranexamic Acid for the Treatment of Acute Epistaxis: A Systematic Review and Meta-analysis. Ann Pharmacotherapy Jun 2019. PMID: 30577703
For More Thoughts on This Topic Checkout:
- First10inEM: Does TXA Work for Everything?
- First10inEM: No Benefit from TXA in Epistaxis?
- SGEM: Times are a Changin for TXA in Epistaxis
- REBEL EM: The NoPAC Trial – TXA for Epistaxis?
Post Peer Reviewed By: Salim Rezaie, MD (Twitter: @srrezaie)
The post Meta-Analysis on Topical TXA for Epistaxis appeared first on REBEL EM - Emergency Medicine Blog.
