
 Background information: Buprenorphine and buprenorphine-containing medications are being used in emergency departments for treatment of patients with opioid use disorder (1). Buprenorphine is a high-affinity partial agonist of the μ-opioid receptor. Buprenorphine has a higher affinity for the μ-opioid receptor than full agonists such as heroin, oxycodone, fentanyl, and methadone. Thus, if a patient is using one of these full agonists and is treated with buprenorphine, buprenorphine may displace the full agonist from the μ-opioid receptor, which may cause precipitated withdrawal. Emergency medicine physicians are likely familiar with precipitated withdrawal caused by naloxone, an opioid receptor antagonist. When a patient withdraws spontaneously from opioids, the withdrawal occurs over a more prolonged time period, but in precipitated withdrawal, the patient experiences severe and sudden-onset symptoms such as tachycardia, diaphoresis, vomiting, diarrhea, dysphoria, and even autonomic instability (2). To avoid precipitated withdrawal, buprenorphine is typically prescribed when the patient either has withdrawal symptoms or has passed through the spontaneous withdrawal period already.
Background information: Buprenorphine and buprenorphine-containing medications are being used in emergency departments for treatment of patients with opioid use disorder (1). Buprenorphine is a high-affinity partial agonist of the μ-opioid receptor. Buprenorphine has a higher affinity for the μ-opioid receptor than full agonists such as heroin, oxycodone, fentanyl, and methadone. Thus, if a patient is using one of these full agonists and is treated with buprenorphine, buprenorphine may displace the full agonist from the μ-opioid receptor, which may cause precipitated withdrawal. Emergency medicine physicians are likely familiar with precipitated withdrawal caused by naloxone, an opioid receptor antagonist. When a patient withdraws spontaneously from opioids, the withdrawal occurs over a more prolonged time period, but in precipitated withdrawal, the patient experiences severe and sudden-onset symptoms such as tachycardia, diaphoresis, vomiting, diarrhea, dysphoria, and even autonomic instability (2). To avoid precipitated withdrawal, buprenorphine is typically prescribed when the patient either has withdrawal symptoms or has passed through the spontaneous withdrawal period already.
Current guidelines are primarily directed in the outpatient setting for initiating buprenorphine. For example, the FDA recommends 8 mg on Day 1 and 16 mg on Day 2 (3). Many patients face barriers to obtaining buprenorphine for reasons including transportation barriers and lack of health insurance coverage. A high-dose buprenorphine ind pathway that rapidly reaches a therapeutic level within hours may help with these barriers to healthcare when compared to the typical multi-day dosing pathway. A higher dose of buprenorphine increases the half-life, may reduce both symptoms of withdrawal/craving, and may be of particular use for patients taking high-dose opioids. Potential risks of high-dose buprenorphine include precipitated withdrawal and buprenorphine overdose. Of note, a recent study from an inpatient psychiatric ward showed that 2 out of 30 patients treated with 96 mg of buprenorphine experienced hypotension (treated with IV fluids), whereas the 60 patients taking either 32 mg or 64 mg did not experience hypotension; additionally, no severe respiratory effects were noted in this study (4). This case series aimed to evaluate whether patients could be treated safely and effectively with high-dose buprenorphine initiated in the emergency department.
REBEL Cast Ep102: High-Dose Buprenorphine Induction in the Emergency Department for Treatment of Opioid Use Disorder
Paper: Herring, A. et al. High-Dose Buprenorphine Induction in the Emergency Department for Treatment of Opioid Use Disorder. JAMA Network Open. 2021. PMID 34264326.
Clinical Question: Can high-dose buprenorphine (>12mg) be safely and effectively initiated in the emergency department for opioid use disorder?
What They Did:
- Retrospective, electronic health record case series of emergency department encounters in Oakland, California of adults treated with sublingual buprenorphine, where providers had received previous training on a standard and high-dose buprenorphine pathway (391 patients with 579 unique encounters).
- Patients who met the buprenorphine pathway criteria were treated with 4 to 8mg of sublingual buprenorphine based on the Clinical Opiate Withdrawal Score (COWS) and reassessed in 30 to 45 minutes.
- Per the pathway, patients with improvement in symptoms with same-day follow-up available were offered the standard-dose pathway and treated with additional dosing of up to 12 mg until the COWS score was <8 (minimal withdrawal symptoms). They were observed for 30-60 minutes and discharged with a prescription for 16mg buprenorphine daily.
- Patients who did not have complicating medical factors but did have barriers to prompt access to buprenorphine after discharge, high opioid tolerance, and/or COWS score ≥8 after initial dosing were offered the high-dose buprenorphine pathway. In the high-dose pathway, subsequent dosing could be in increments of 8 to 24 mg with 30-60 minute observation after each dose. They were observed for 30-60 minutes and discharged with a prescription for 16mg buprenorphine daily.
Inclusion:
- ≥ 18 years of age
- Presented to the emergency department between January 1st, 2018 and December 31st, 2018 with uncomplicated opioid withdrawal
- Patients determined to be clinically appropriate for emergency department initiation of buprenorphine based on clinical history, vital signs, physical examination, COWS score (≥8 either upon presentation or after reassessment)
Exclusion:
- Although not true exclusion criteria from the case series, based on the ED protocol, possible exclusion criteria from the protocol included complicating factors for which patients were not appropriate for the high-dose buprenorphine pathway, including age >65, recent methadone use, viable pregnancy, co-ingestant use, recent naloxone treatment, and other severe medical illness, and these patients were included if buprenorphine was initiated based on recommended addiction specialist consultation
Outcomes:
-
Primary:
- Precipitated withdrawal rate, defined based on clinical diagnosis or increasing COWS scores within 1 hour following buprenorphine treatment
-
Serious adverse events attributable to buprenorphine including sedation, hypoxia, decreased respiratory rate, and naloxone dosing requirement in the ED or within 24 hours after discharge
- Serious adverse events were characterized as life-threatening, urgent intervention required or death
-
Secondary:
- Other vital signs including maximum and minimum heart rate and systolic blood pressure, oxygen saturation
- Other non-serious adverse events
- Length of stay in the Emergency Department
Results:
- Included 391 patients with 579 unique encounters:
-
366 encounters with high-dose buprenorphine (63.2%), defined as >12mg sublingual buprenorphine
- 23.8% ≥ 28mg
- 213 encounters with standard-dose buprenorphine (38.8%), defined as ≤12mg sublingual buprenorphine
- Median age = 36 years (Range 29 to 48)
- Homeless = 22.5%
- Comorbid non-substance use-related psychiatric disorders = 41.2%
- Never been treated with buprenorphine = 53.5%
-
No serious adverse events were associated with buprenorphine:
- No hypoxia, sedation, or treatment with naloxone after buprenorphine administration
-
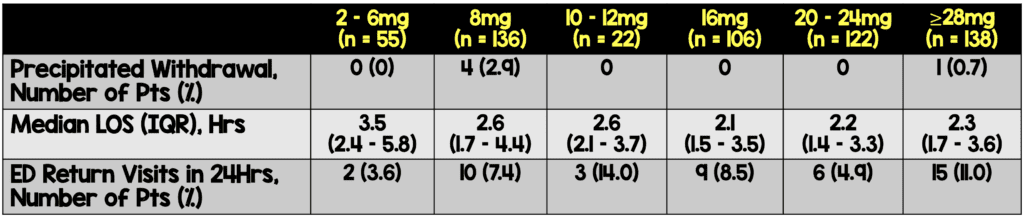
Precipitated withdrawal occurred in 5 cases (0.8%):
- Precipitated withdrawal occurred after 8 mg dosing in 4 of the 5 cases, and these cases responded to treatment with further buprenorphine dosing for a total of 28 mg
- Precipitated withdrawal occurred in the fifth case after a second 24 mg following the first 8 mg dose, although this patient had stimulant use as well
- No patients required admission for precipitated withdrawal, and no cases of life-threatening adverse events were determined to be related to buprenorphine
- All patients requiring oxygen were started on oxygen prior to initiation of buprenorphine
-
There was no clear trend between nausea and vomiting rates with increased buprenorphine doses:
- Documented incidence of nausea or vomiting after buprenorphine was low (2 to 6% of cases)
- 8 mg = 4%
- 10 to 12 mg = 6%
- 16 mg = 5%
- 20 to 24 mg = 2%
- ≥28 mg = 2%
- The median length of stay in the Emergency Department was 2.4 hours, with a lower length of stay in the high-dose groups
- A high percentage of patients in the ≥28 mg of buprenorphine dosing returned to the emergency department within 24 hours, although none had adverse events related to buprenorphine (10 to 18% were unsuccessful accessing follow-up treatment immediately after discharge and required repeat dosing in the ED)
Strengths:
- Large case series of a high-dose buprenorphine pathway in the emergency department, for which data is lacking
- Provides further information on treatment of precipitated withdrawal with buprenorphine (precipitated withdrawal was successfully treated with higher buprenorphine doses in 4 out of 5 patients, and this strategy has been reported previously in case studies) (5)
- Asks a clinically important question addressing withdrawal symptoms and barriers to short-term medication access
- Assesses an important issue of inadequate dose escalation on initiation as this could be a reason that patients stop receiving treatment
- The high-dose pathway was widely accepted and adopted by a large proportion of ED clinicians
- ED length of stay was remarkably short with a median of just over 2 hours
- Shows that an alternative, less rigid, more rapid pathway for a complex patient population is needed to meet patients needs
- Potential adverse events were reviewed by a clinician who was independent of any clinical site
Limitations:
- The providers in this case series received extensive training in addiction medicine for opioid use disorder due to being a site associated with the California Bridge Program. Further training and experience may be necessary for emergency physicians to become comfortable with initiating high-dose buprenorphine.
- This is a retrospective case series and not a randomized control trial. Further research is warranted for patients meeting criteria for the high-dose pathway to be randomized into a high and standard-dose group.
- Statistical significance was calculated with all pairwise comparisons performed for six buprenorphine categories if the omnibus test was significant, but there was no adjusting for multiple comparisons, and this was explained as attempting to show potential safety concerns by not adjusting for type 1 error.
- Demographic data were not provided for whether the patient was treated with the high-dose or standard-dose pathway, and there likely were significant differences for multiple reasons including social factors being included in the pathway.
- Although no patients experienced hypoxia after buprenorphine, other adverse events were not specifically addressed (such as hypotension). They were only addressed by a median and interquartile range for minimum blood pressure.
- Opioid use disorder includes multiple subcategories with different durations of action and methods of intake, and it would have been helpful to have more details, as this is hypothesized to affect buprenorphine dosing requirements.
- COWS scores were not documented on all patients, and therefore no comparison of initial and subsequent COWS scores were available for analysis.
- Only the monoproduct of buprenorphine was used in this study. It is possible that high-dose induction using a combination buprenorphine-naloxone formulation could result in clinically significant outcomes, although sublingual naloxone bioavailability is very low due to first-pass metabolism (6)
Discussion: In this case series, buprenorphine in high doses (defined as >12mg) was safe and effective in treating opioid use disorder in patients presenting in active withdrawal. Low rates of precipitated withdrawal were observed with no relationship to treatment with a high dose of buprenorphine. Possible explanations of precipitated withdrawal include the use of longer-acting opioids such as methadone, which has been associated with precipitated withdrawal. All patients who required oxygen were started on oxygen prior to initiation of buprenorphine. There was no clear trend between nausea and vomiting rates with increased buprenorphine doses. Lengthy emergency department times were not required for standard or high-dose treatment, with the median length of stay in the Emergency Department of 2.4 hours. A high percentage of patients in the ≥28 mg of buprenorphine dosing returned to the emergency department within 24 hours, although none had adverse events related to buprenorphine. This difference was likely due to the high-dose patients being selected for the pathway due to more significant symptoms and social factors making follow-up difficult. Return visits are likely going to vary across the United States based on the availability of follow-up resources for substance use.
Overall, this is a very encouraging case series on the safety and efficacy of a high-dose emergency department buprenorphine pathway for patients with opioid use disorder in select patients who would be likely to benefit from high-dose treatment, although caution must be exercised given that this is a case series and not an experimental study. The high-dose buprenorphine pathway is not appropriate for all patients, and a balance between potential risks such as oversedation and hypoxia versus benefits needs to be made. An experimental study would be useful to explore the potential benefits of a higher-dosing pathway and to evaluate which patient factors are associated with benefit in a higher-dosing in the emergency setting.
Author conclusion: “These findings suggest that high-dose buprenorphine induction, adopted by multiple clinicians in a single-site urban ED, was safe and well-tolerated in patients with untreated OUD. Further prospective investigations conducted in multiple sites would enhance these findings.“
Clinical take-home point: Based on this retrospective case series, in the appropriate patients, use of a high dose of the monoproduct of buprenorphine was shown to not be associated with precipitated withdrawal, oversedation, or other adverse events. Physicians may consider high-dose buprenorphine initiation for patients with opioid use disorder experiencing withdrawal symptoms, although further research including an experimental study is needed.
References:
- Herring, A. et al. High-Dose Buprenorphine Induction in the Emergency Department for Treatment of Opioid Use Disorder. JAMA Network Open. 2021. PMID 34264326.
- Herring, A. et al. Managing Opioid Withdrawal in the Emergency Department with Buprenorphine. Ann Emerg Med. 2019. PMID 30616926.
- Substance Abuse and Mental Health Administration. TIP 63: Medications for Opioid Use Disorder. May 2020. [Link is HERE]
- Ahmadi J et al. Single High-Dose Buprenorphine for Opioid Craving During Withdrawal. Trials 2018. PMID 30526648
- Oakley B et al. Managing Opioid Withdrawal Precipitated by Buprenorphine with Buprenorphine. Drug Alcohol Rev. 2021. PMID 33480051
- Blazes C et al. Reconsidering the Usefulness of Adding Naloxone to Buprenorphine. Front Psychiatry 2020. PMID 33061915
For More Thoughts on This Topic, Check Out:
- The Tox and the Hound (EMCrit): We Have a MOUD* Disorder
- American Academy of Emergency Medicine: Management of Opioid Use Disorder in the Emergency Department: A White Paper Prepared for the American Academy of Emergency Medicine
- Annals of Emergency Medicine: Consensus Recommendations on the Treatment of Opioid Use Disorder in the Emergency Department
- Emergency Medicine Residents’ Association (EMRA): Buprenorphine Initiation in the ED and MAT
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post REBEL Cast Ep102: High-Dose Buprenorphine Induction in the Emergency Department for Treatment of Opioid Use Disorder appeared first on REBEL EM - Emergency Medicine Blog.
