
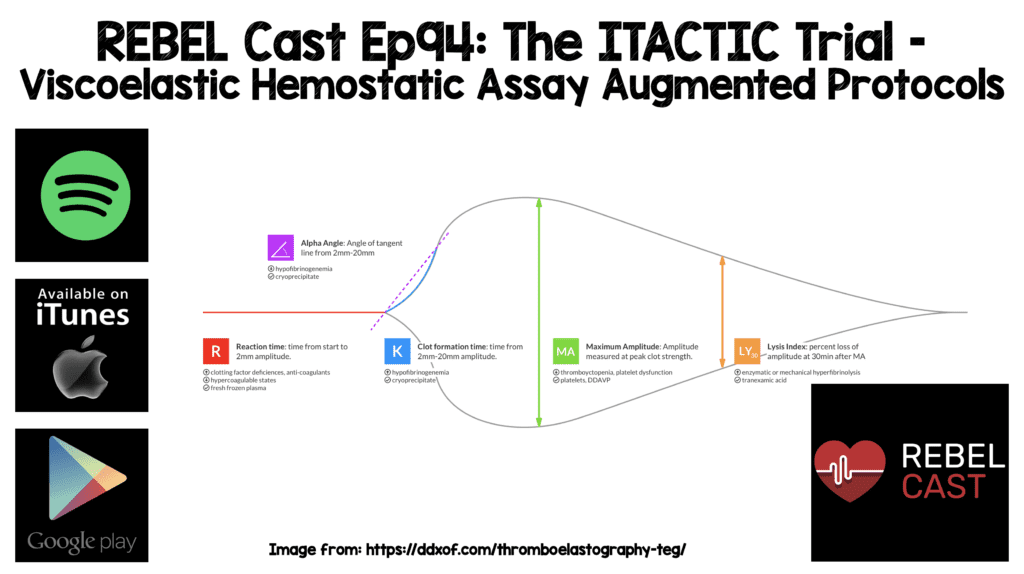
 Background: Current trauma resuscitation prioritizes control of bleeding and uses massive transfusion protocols to prevent and treat coagulopathy. This is typically done in the form of massive transfusion protocols delivered in proportions that approach the composition of whole blood. Two strategies to help guide this replacement of blood products are conventional coagulation tests and viscoelastic hemostatic assays.
Background: Current trauma resuscitation prioritizes control of bleeding and uses massive transfusion protocols to prevent and treat coagulopathy. This is typically done in the form of massive transfusion protocols delivered in proportions that approach the composition of whole blood. Two strategies to help guide this replacement of blood products are conventional coagulation tests and viscoelastic hemostatic assays.
REBEL Cast Ep94: The ITACTIC Trial – Viscoelastic Hemostatic Assay Augmented Protocols
Special Guest:

Ken Milne, MD
South Huron Hospital Association
Website: The SGEM
Twitter: @TheSGEM
Paper: Badsaas-Aasen K et al. Viscoelastic Haemostatic Assay Augmented Protocols for Major Trauma Haemorrhage (ITACTIC): A Randomized, Controlled Trial. Intensive Care Med 2020. PMID: 33048195
Clinical Question: Does augmenting massive transfusion protocols using viscoelastic hemostatic assays (VHAs) decrease mortality and massive transfusion at 24 hours compared to conventional coagulation tests (CCTs)?
What They Did:
- Implementing Treatment Algorithms for the Correction of Trauma-Induced Coagulopathy (ITACTIC)
- Pragmatic, multicenter, randomized controlled trial of trauma patients who received empiric massive transfusion protocols augmented by:
- Viscoelastic Hemostatic Assays (VHAs)
- Conventional Coagulation Tests (CCTs)
- Conducted at 7 major trauma centers in Europe
- All enrolled patients received:
- Empiric tranexamic acid
- Blood components delivered in a 1:1:1 ratio of RBCs, plasma and platelets (Covered on the SGEM #109)
- Limited infusion of crystalloid fluids
- Important Definitions:
- Time of Hemostasis: 1h after last RBC transfusion was given and treating clinicians stated hemostasis had been achieved
- Massive Transfusion: Administration of ≥10 units of PRBCs in the 1st 24hrs after injury (While this is a common definition, LITFL has used other parameters)
- One pool of cryoprecipitate = 2 grams of fibrinogen concentrates
- One pool of platelets = 4 individual platelet units
Outcomes:
- Primary: Proportion of patients alive and free of massive transfusion at 24h after injury
- Secondary Outcomes of Note (19 secondary outcomes):
- All-cause mortality at 6h, 24h, 28d, and 90d
- Total blood components
- 28d ventilator-free
- ICU free days
- Total hospital length of stay
- Proportion of patients with symptomatic thromboembolic events
- Proportion of patients with multiple organ dysfunction
- Proportion of patients with serious adverse events
Inclusion:
- Adult (16 years of age and older) trauma patients presenting with hemorrhagic shock at any time from injury to admission to the ED
- Hemorrhagic shock defined by HR >100 BPM and/or SBP <90mmHg AND activation of the local massive transfusion protocol
- Clinical signs of bleeding activating massive transfusion protocol
- RBC transfusion initiated
- Randomized within 3hrs of injury and maximum of 1hr after admission to the emergency department
Exclusion:
- If any inclusion criteria were not met
Results:
- 480 patients eligible
- 411 randomized
- 396 patients in the intention-to-treat (ITT) analysis
- 2/3rds had blunt trauma alone
- Median Injury Severity Score (ISS) = 26 (Range 17 to 36)
- Median time from injury to admission = 69min
- Proportion of Patients Alive and Free of Massive Transfusion at 24h After Injury:
- VHA: 67%
- CCT: 64%
- OR 1.15; 95% CI 0.76 to 1.73
- In the VHA arm 14% of patients died and 26% received a massive transfusion at 24h
- In the CCT arm 17% of patients died and 28% received a massive transfusion at 24h
- Per-protocol analysis excluded 83 patients and found no statistically significant difference between groups
- No statistical difference in other secondary outcomes
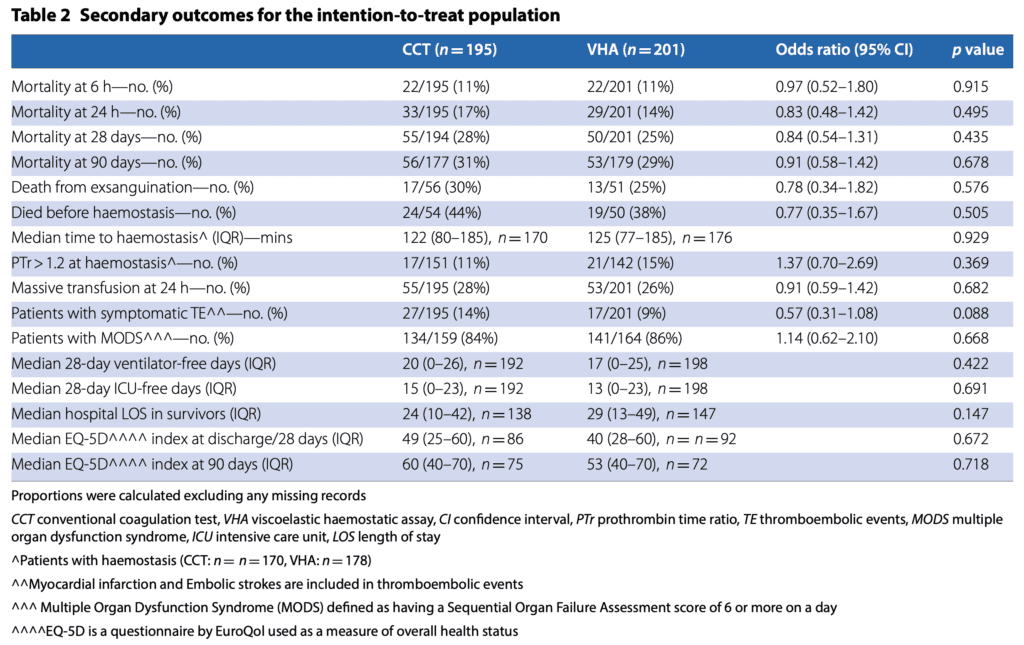
- QOL on the EQ-5D at 28 and 90d were not statistically different, but trends were worse in the VHA group (0 the worst possible health status to 100 the best)
- Both were worse in the VHA group
- 28d: CCT 49 vs VHA 40
- 90d: CCT 60 vs VHA 53
- No statistical difference in any adverse events
- There was a 10% absolute difference in thrombotic events (i.e. clinically significant)
- VHA: 14%
- CCT: 24%
- There was a 10% absolute difference in thrombotic events (i.e. clinically significant)
- Both were worse in the VHA group
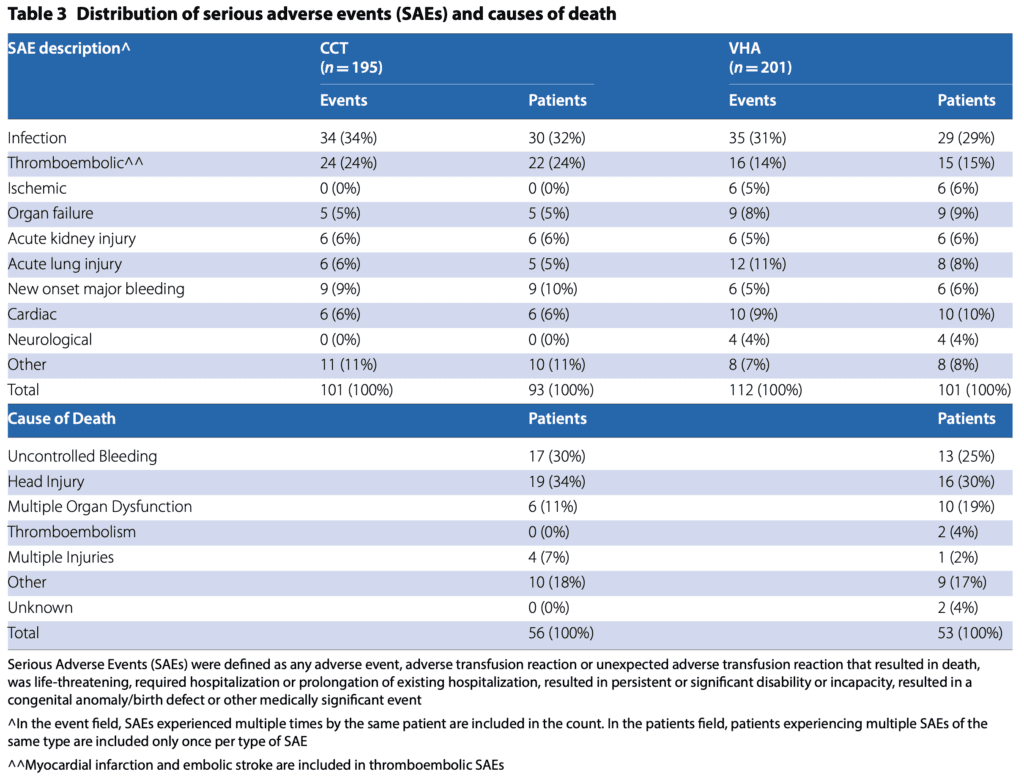
-
-
- 28d Mortality:
- VHA: 25%
- CCT: 28%
- OR 0.84; 95% CI 0.54 to 1.31
- Alive and Free of Massive Transfusion at 24h After Injury in Prespecified Subgroup of Patients with TBI (74pts)
- VHA: 64%
- CCT: 46%
- OR 2.12; 95% CI 0.84 to 5.34
- 28d Mortality:
-
Strengths:
- Multicenter, randomized clinical trial asking an important question in trauma management
- Used prior data to estimate the number of patients requiring massive transfusion by 24h to calculate sample size
- Used an intention-to-treat and per-protocol to analyze all primary and secondary outcomes
- Baseline characteristics were fairly well matched between treatment groups.There were some differences, but whether or not these would have a statistical or clinical impact is unknown
Limitations:
- Trial was unblinded to treating clinical teams potentially causing bias. If physicians thought that VHA was superior, then the results should have been towards finding superiority.The fact they did not strengthens the conclusions
- Very few patients in this trial had baseline coagulopathy or developed coagulopathy before hemostasis
- Only ≈7% of patients were on oral anticoagulants in this trial and definitive conclusion on which strategy is better in these patients cannot be determined from this study
- Lower than predicted difference in effect size between groups of 3% (predicted 13%)
- Study performed at large trauma centers with experience in the use of VHA devices which may not be the case in centers that do not see trauma frequently or don’t have VHA available
- In Ken’s shop they do not have VHA testing and only have 2 units of 0 negative blood and some TXA.Therefore, the results of this trial would not have external validity to critical access hospitals with limited resources. The goal therefore would be to get these trauma patients to the level 1 trauma centers ASAP
- Baseline differences (age 3 years, gender 9%, prior anticoag 2%, severe TBI 2% or received TXA 4%) were not adjusted for in the analysis. These differences could just be a result of randomization, but still can impact the results
Discussion:
- Authors were looking for a 15% difference in the reduction in their primary outcome with VHA compared to CCT
- Per Protocol Group Excluded:
- Did not have at least one VHA or CCT test performed
- Did not meet inclusion criteria
- Received the wrong test
- Died within 60min after baseline blood sampling
- Achieved hemostasis within 60min of baseline sampling
- Interesting Facts:
- After enrollment and before hemostasis 67% of patients in the VHA group and 36% of patients in the CCT group had received a study intervention (post hoc analysis)
- Study intervention was given a median of 21 min earlier in the VHA group (61min vs 80min) (post hoc analysis)
- Receiving study intervention within 3h of injury: VHA 64% vs CCT 45%
- Between baseline and hemostasis patients in the VHA arm received more fibrinogen supplementation (median fibrinogen equivalent dose = 4g vs CCT = 0g)
- These differences of quicker actually strengthen the confidence in accepting the null hypothesis of no superiority of VHA vs CCT
- The 28-mortality benefit seen with VHA vs CCT in the TBI subgroup of patients is subgroup analysis of a secondary outcome.This may have been random chance, is hypothesis generating, and requires further study to confirm
- An important point to highlight is that blood products are a precious resource.VHA-augmented resuscitation identified more coagulation deficits than CCT guided resuscitation. However, this resulted in 1.8 times more products than the CCT group with no benefit in the proportion of patients alive and free of massive transfusion at 24h after injury or in any of the secondary mortality outcomes
Author Conclusion: “There was no difference in overall outcomes between VHA- and CCT-augmented-major haemorrhage protocols.”
Clinical Take Home Point: It is unsurprising that coagulation monitoring did not alter clinical outcomes in this study as there was a high prevalence of patients who never had or developed coagulopathy. This RCT does not provide evidence to support using VHA to guide resuscitation in adult trauma patients.
References:
- Badsaas-Aasen K et al. Viscoelastic Haemostatic Assay Augmented Protocols for Major Trauma Haemorrhage (ITACTIC): A Randomized, Controlled Trial. Intensive Care Med 2020. PMID: 33048195
For More Thoughts on This Topic Checkout:
- The Bottom Line: ITACTIC
- St. Emlyn’s Blog: Blood Products in Trauma – What’s the Best (I)TACTIC?
Post Peer Reviewed By: Ken Milne, MD (Twitter: @TheSGEM)
The post REBEL Cast Ep94: The ITACTIC Trial – Viscoelastic Hemostatic Assay Augmented Protocols appeared first on REBEL EM - Emergency Medicine Blog.

