
 Background Information:
Background Information:
Diabetic Ketoacidosis (DKA) is a life-threatening complication of diabetes that we frequently encounter in both the emergency department (ED) and intensive care unit (ICU). While intravenous fluid replacement remains one of several cornerstones of therapies, much debate exists on exactly which IV fluid results in faster DKA resolution with less adverse events. While international guidelines recommend 0.9% sodium chloride as the fluid of choice, it has been associated with hyperchloremia and prolonged ICU length of stays.1 Plasmalyte on the other hand has less chloride but additional acetate and gluconate anions which can serve as precursors for acetoacetate, a ketone body that is eventually converted to beta-hydroxybutyrate. The authors of the following study sought to investigate whether plasmalyte would lead to faster DKA resolution without increasing ketone generation.
Paper:
Ramanan M, et al. Sodium chloride or Plasmalyte-148 evaluation in severe diabetic ketoacidosis (SCOPE-DKA): a cluster, crossover, randomized, controlled trial. Intensive Care Med. 2021 Oct 5. PMID: 34609547
Clinical Question:
- Does the administration of plasmalyte result in faster resolution of critically ill patients with severe DKA and not increase ketone generation compared to the administration of sodium chloride?
What They Did:
- Cluster, cross-over, open-label randomized control phase 2 trial
- Conducted in seven rural and metropolitan Australian intensive care units over a 13-month period
- The time period was divided into two 6-month intervention periods with a 1-month washout period in the middle
- Participants were randomized into one of the following two groups for the first intervention period of six months:
- Sodium Chloride (SC) group
- Plasmalyte (PL) group
- The sites then crossed-over to administer the other fluid during the second intervention period of 6 months
- Fluid compliance was defined as the proportion of the total fluid received by each patient that was consistent with the allocation (ex. Patient in PL group received total of 5L, if 4L was PL then compliance was 80%)
- Point-of-care biochemical analyses were also performed at individual sites to evaluate ketone concentrations and measure beta-hydroxybutyrate
Inclusion Criteria:
- All patients >16 years old who presented to the ED or ICU with severe DKA defined as the following:
- Arterial pH < 7.25 (or serum bicarbonate < 15 mmol/L)
- Blood glucose > 14 mmol / L (or >250 mg/dL)
- Requiring ICU admission based on judgement of treating clinician
Exclusion Criteria:
- Under the age of 16 years old
- Previously included in this trial
- Contraindication to fluid administration
- Suspected diagnosis of hyperosmotic hyperglycemic nonketotic syndrome
Outcomes:
Primary
- Change in base excess to > -3 mEq/L at 48-hours post ICU admission
- Sensitivity analyses for primary outcome were the following:
- Change in base excess to > -3 mEq/L at 24-hours post ICU admission
- DKA resolution at 24-hours post ICU admission per American Diabetes Association criteria:
- Plasma glucose < 11.1 mmol/L and two of the following:
- Bicarbonate > 15 mmol/L
- Venous pH > 7.3
- Anion gap < 12 mEq/L
Secondary
- ICU mortality and length of stay
- Hospital mortality and length of stay
- Organ support (ie. invasive and non-invasive ventilation, CRRT)
- Cumulative urine output and fluid balance at 24-, 48- and 72-hours post-ICU admission
- Biochemical outcomes (ie. serum potassium, chloride, pH, pCO2, base excess, anion gap, blood glucose and ketone concentrations) at 6-, 12-, 18-, 24-, 36-, 48- and 72-hours post-ICU admission
Safety
- Proportion of patients with any of the following within 24 hours post-ICU admission
- Severe hypokalemia (K+ < 3.0 mol/L)
- Hypoglycemia (Glucose < 3.8 mmol/L)
- Hypophosphatemia (Phosphate < 0.7 mmol/L)
- Hypocalcemia (iCa < 1 mmol/L)
- Persistent ketosis (Ketone concentration > 1.5 mmol/L)
Results:
Critical Results:
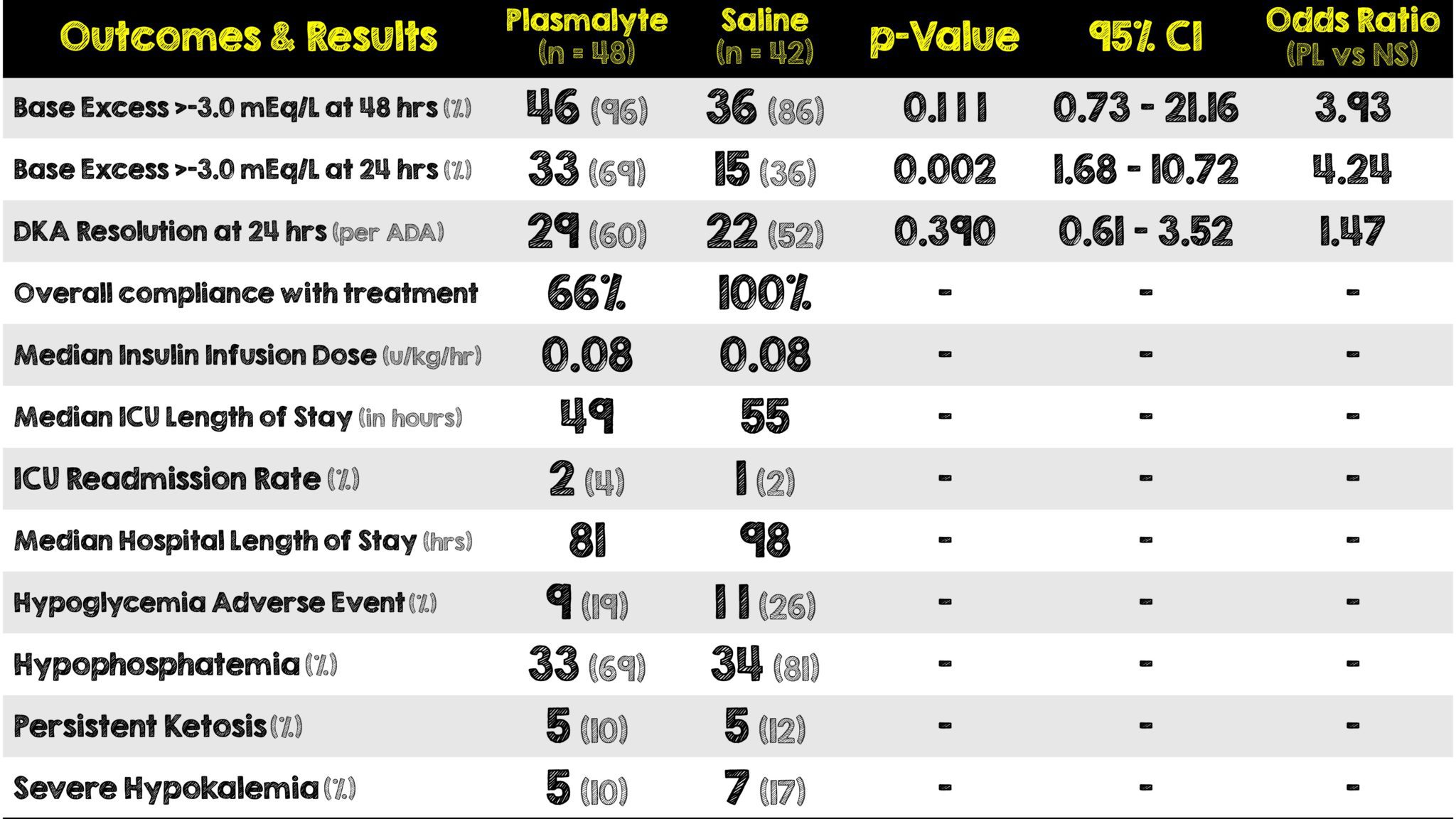
- Mean volume of fluid administered up to 48 hours post ICU-admission was 6798 mL in the PL group and 6574 mL in the SC group.
- Compliance with treatment allocation to plasmalyte was greater in the ICU (76%) compared to the ED (50%)
Strengths:
- Conducted across multiple ICUs in different settings (ie. rural and metropolitan)
- First study investigating ketone and beta-hydroxybutyrate concentrations as potential adverse effects of plasmalyte
- Study fluid was used for all resuscitation and IV maintenance purposes
- Kept all aspects of DKA management similar across both groups leaving fluid type as the only management stipulated by the trial
- Cluster-crossover design utilized to reduce setup and education costs, and easier delivery of the trial intervention as a fluid choice
- Results similar to subgroup analysis of 2 larger cluster RCTs evaluating balanced crystalloids vs SC in ED and ICU patients
- Utilized an objective and well-regarded means of determining DKA resolution
- Only two patients enrolled were lost to follow-up and did not have any treatment or outcome data
Limitations:
- Phase 2 trial not powered to detect differences in clinical outcomes (ie. small sample size) and results can only be considered exploratory
- Open-label study potentially introduces bias
- An ICU-based and not ED-based study
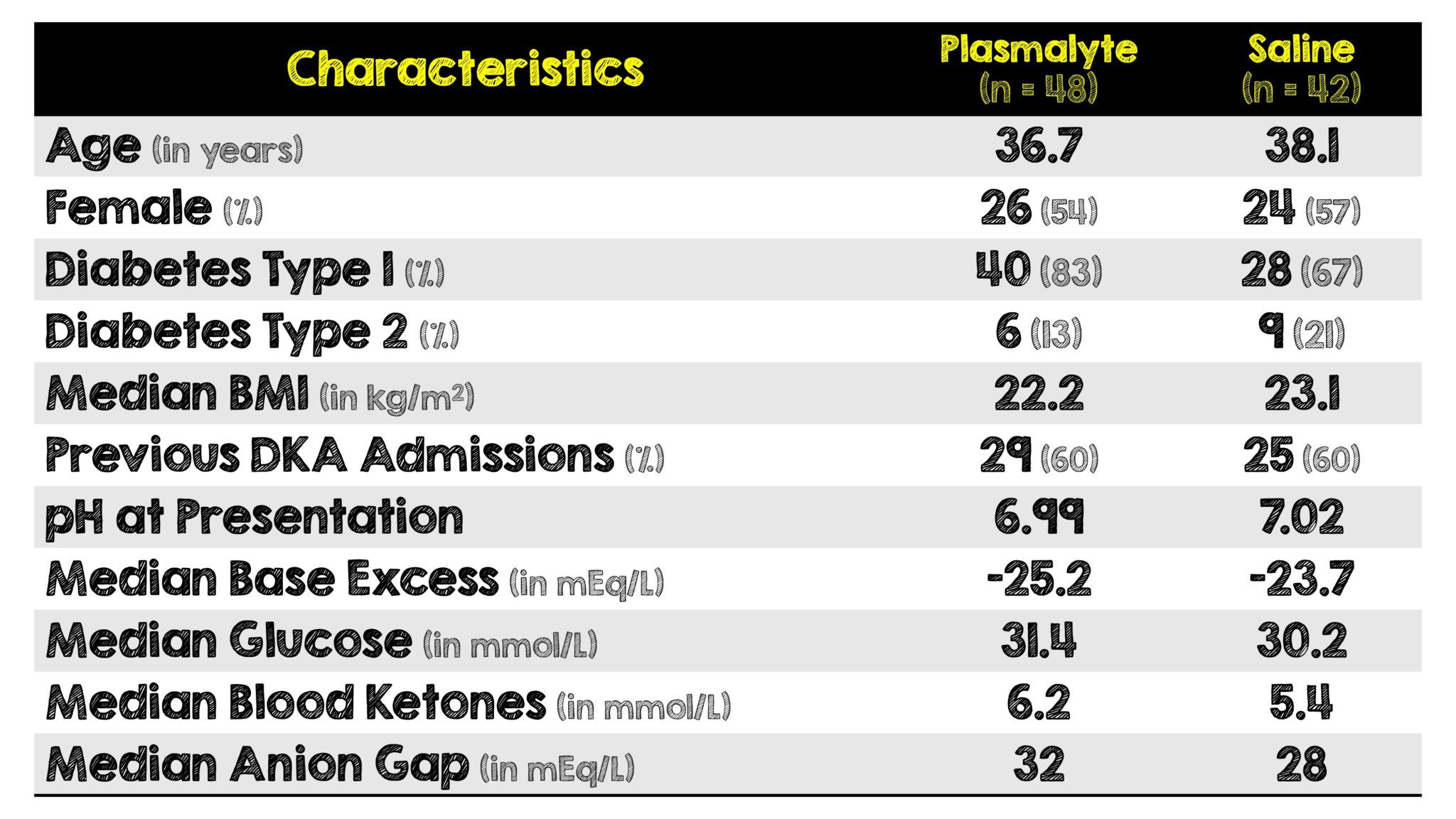
- Baseline imbalances existed between the two groups with more patients in the plasmalyte group having:
- Type I DM (83% vs 67%)
- Higher initial AG (32 vs 28 mEq/L)
- Higher initial blood ketone concentrations (6.2 vs 5.4 mmol/L)
- Low adherence to allocated fluids in the PL group and substantial volumes of SC were used, likely due to use of SC for drug dilution
- Volume and rate of administration of fluids were guided by clinical and biochemical endpoints determined by treating clinician which introduces confounding variability
- No third arm administering LR to more thoroughly compare all three fluids
Discussion:
- This is the first trial comparing plasmalyte to normal saline in regard to DKA resolution and specifically looking at the potential adverse effects of plasmalyte.
- The authors justify this being a cluster, cross-over study for the purposes of reduced setup, education costs and ease of intervention delivery. However due it’s small sample size and lack of power, the results have to be cautiously interpreted and was not able to detect differences in clinical outcomes.
- With that said, the study’s differing institutions of enrollment (ie. rural vs metropolitan hospitals) helps add to a variable patient population.
- Furthermore, depending on the institutions (particularly in the United States), plasmalyte can be expensive and not readily available. This cost factor heavily dictates which emergency departments or intensive care units even stock plasmalyte compared to the inexpensive normal saline. The current estimated cost of 1L of plasmalyte is more than double than that of 1L normal saline.2
- As studies like this get produced we learn more about the solutions we should be using. In addition to no association with ketone generation, the lack of other adverse effects such as hypoglycemia and hypophosphatemia noted in this trial, make the use of plasmalyte more attractive when compared to normal saline.
- The authors of this study unfortunately missed the opportunity of adding a third arm comparing plasmalyte, normal saline and lactated ringers. This would have been even more informative regarding which specific fluids expedite the resolution of DKA.
- Lastly, DKA patients often require multiple boluses of fluid. We’ve talked at length about normal saline here on REBEL and even had in person debates about it. The patients in this study were not undifferentiated. It’s important to note that high volumes of normal saline can potentially make their metabolic acidosis worse by inducing hyperchloremia and therefore should be used with caution.
Author’s Conclusions:
- Plasmalyte-148, compared to sodium chloride 0.9%, may lead to faster resolution of metabolic acidosis in patients with DKA without an increase in ketosis. These findings need confirmation in a large, Phase 3 trial.
Our Conclusion:
- Although a small cluster-crossover trial that was too underpowered to detect a difference in clinical outcomes, this paper provides us additional valuable information on the effects and adverse events related to plasmalyte. More importantly, this trial helps pave the way and open the discussion for a larger confirmatory trial to compare these two solutions (and possibly a third being lactated ringers). The financial cost of plasmalyte may be the driving factor limiting availability and ultimately use.
Clinical Bottom Line:
- Where available and financially feasible, the use of plasmalyte solution instead of normal saline may be associated with faster resolution of metabolic acidosis from DKA, no increase in ketone generation and with less adverse effects. However, the results of this underpowered small cluster crossover trial need to be interpreted cautiously until a larger phase 3 trial can be performed.
REFERENCES:
- Ramanan M, et al. Sodium chloride or Plasmalyte-148 evaluation in severe diabetic ketoacidosis (SCOPE-DKA): a cluster, crossover, randomized, controlled trial. Intensive Care Med. 2021 Oct 5. PMID: 34609547
- Kwong YD, Liu KD. Selection of Intravenous Fluids. Am J Kidney Dis. 2018; PMID: 29980374
For More Thoughts on This Topic Checkout:
- REBEL EM: Is the Great Debate Between Balanced vs Unbalanced Crystalloids Finally Over?
- REBEL EM: Rebellion in EM 2019: What the Fluid? Wieters vs Bryant
Post Peer Reviewed By: Salim Rezaie, MD (Twitter: @srrezaie)
The post SCOPE-DKA: Normal Saline vs Plasmalyte in Severe DKA appeared first on REBEL EM - Emergency Medicine Blog.
