Background: Saline (0.9% sodium chloride) has historically been one of the most common intravenous fluids administered in critically ill adults. However, the supraphysiologic chloride concentration can cause hyperchloremia, metabolic acidosis, renal vasoconstriction and alter immune function. There is nothing normal about normal saline. Balanced crystalloids (i.e. lactated Ringer’s solution, Plasma-Lyte A, etc) contain electrolyte compositions that are closer to physiologic levels. Recently, the Isotonic Solutions and Major Adverse Renal Events Trial (SMART) [2] compared balanced crystalloids to saline among critically ill adults and found that balanced crystalloids decreased the composite outcome of death, new renal replacement therapy, or persistent renal dysfunction (This composite outcome was primarily driven by mortality benefit). Interestingly, in the subgroup analyses of septic patients, balanced crystalloids seemed to have its biggest benefit in MAKE30 compared to saline.
Background: Saline (0.9% sodium chloride) has historically been one of the most common intravenous fluids administered in critically ill adults. However, the supraphysiologic chloride concentration can cause hyperchloremia, metabolic acidosis, renal vasoconstriction and alter immune function. There is nothing normal about normal saline. Balanced crystalloids (i.e. lactated Ringer’s solution, Plasma-Lyte A, etc) contain electrolyte compositions that are closer to physiologic levels. Recently, the Isotonic Solutions and Major Adverse Renal Events Trial (SMART) [2] compared balanced crystalloids to saline among critically ill adults and found that balanced crystalloids decreased the composite outcome of death, new renal replacement therapy, or persistent renal dysfunction (This composite outcome was primarily driven by mortality benefit). Interestingly, in the subgroup analyses of septic patients, balanced crystalloids seemed to have its biggest benefit in MAKE30 compared to saline.
What They Did:
- Compare the effect of balanced crystalloids versus saline on 30d in-hospital mortality among critically ill adults with sepsis
- Secondary analysis of patients from the Isotonic Solutions and Major Adverse Renal Events Trial (SMART) admitted to the medical ICU with a diagnosis of sepsis
- The SMART trial was a non-blinded, cluster-randomized, multiple-crossover trial among critically ill adults admitted to 5 ICUs at Vanderbilt University
- Patients enrolled received either the clinician’s choice of lactated Ringer’s solution or Plasma-Lyte A vs saline (0.9% sodium chloride) for intravenous fluid
Outcomes:
- Primary: 30d in-hospital mortality
- Secondary:
- 60d in-hospital mortality
- ICU-free days
- Ventilator-free days
- Vasopressor-free days
- Renal replacement therapy-free days
- Proportion of patients with major adverse kidney events within 30d (MAKE30) – Defined as death, new receipt of RRT or persistent renal dysfunction at the 30d
Inclusion:
- Adult patients
- Diagnosis of sepsis
- Admitted to medical ICU
Exclusion:
- Age <18 years
- Transferred from outside hospitals
Results:
- Of 15,802 patients enrolled in SMART trial, 1641 (10.4%) patients were admitted to the medical ICU with a diagnosis of sepsis
- Cumulative Volume Through Day 3
- NS = 1935 +/- 2873mL
- BC = 1872 +/- 2744mL
- Median Volume Administered
- BC = 1000mL (Range 0 – 3210)
- NS = 1020mL (Range 0 – 3500)
-
30d In-Hospital Mortality:
- Balanced Crystalloids: 26.3%
- Saline: 31.2%
- aOR 0.74; 95% CI 0.59 – 0.93; p = 0.01
- NNT = 20
- Major Adverse Kidney Events Within 30d (MAKE30):
- Balanced Crystalloids: 35.4%
- Saline: 40.1%
- aOR 0.78; 95% CI 0.63 – 0.97
- Vasopressor-Free Days
- Balanced Crystalloids: 20 +/- 12
- Saline: 19 +/-13
- aOR 1.25; 95% CI 1.02 – 1.54
- Renal Replacement Therapy-Free Days:
- Balanced Crystalloids: 20 +/- 12
- Saline: 19 +/- 13
- aOR 1.35; 95% CI 1.08 – 1.69
Strengths:
- Used mortality as the primary outcome which is a patient oriented outcome
- Prespecified subgroup analysis
- Baseline characteristics were well balanced between groups
- Confirmed the result of the primary outcome with numerous sensitivity analyses
Limitations:
- Secondary analysis of a larger clinical trial making the results at risk of a type I error
- All patients were enrolled from a single academic center
- Fluid randomization was not blinded
- Primary analysis was based on ICD-10 codes as a surrogate for sepsis
- Differences in ventilator-, vasopressor-, and renal replacement therapy-free days are sensitive to differences in in-hospital mortality between groups
- Also look at limitations of the SMART trial as they apply here
Discussion:
- 91% of patients in the balanced crystalloid group received lactated Ringer’s solution, essentially making this a LR vs NS comparison. We cannot extrapolate these results to other balanced crystalloids such as Plasma-Lyte A
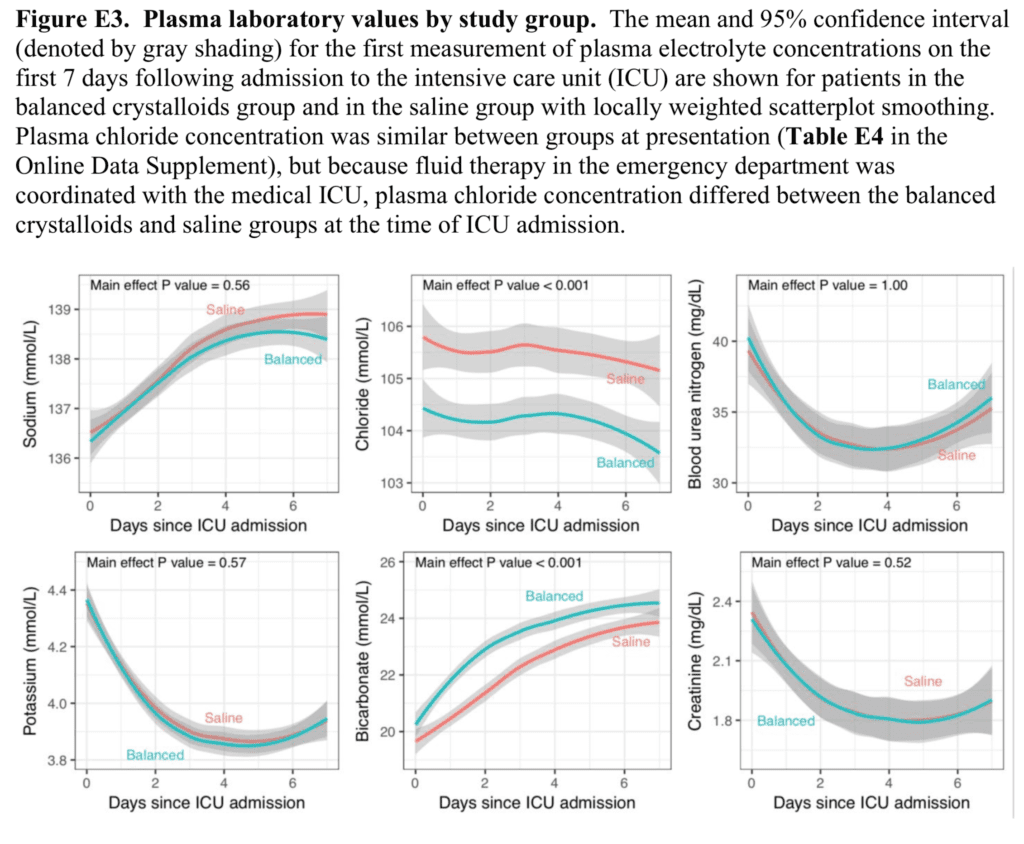
- Serum Electrolyte Levels by Solution
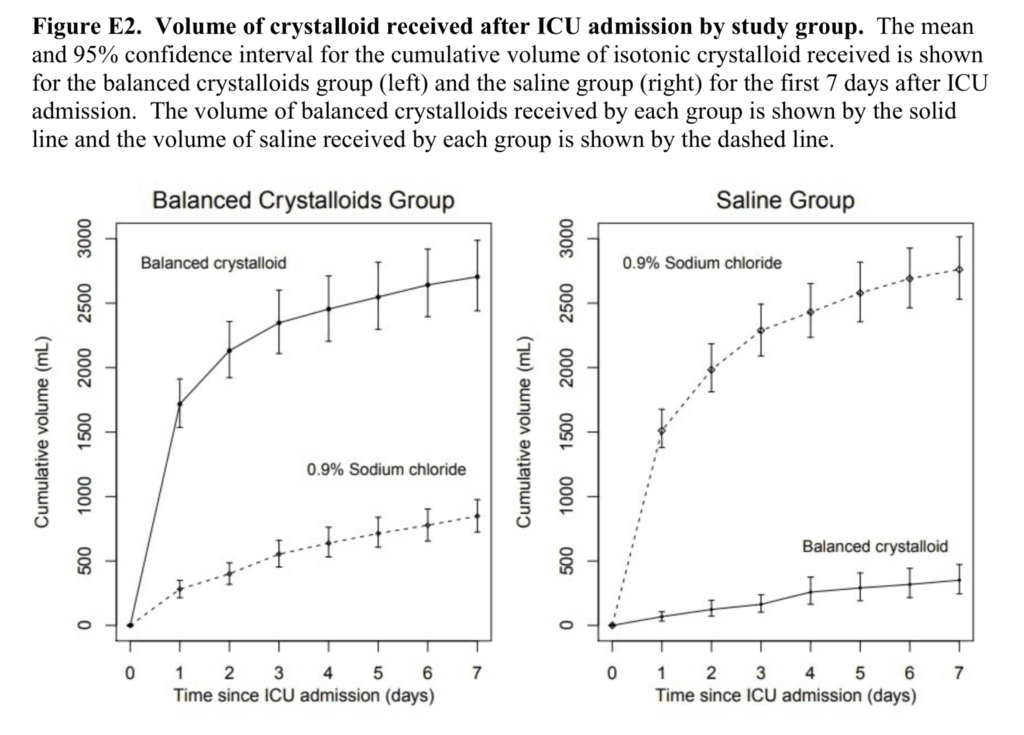
- Volume of Fluid After ICU Admission
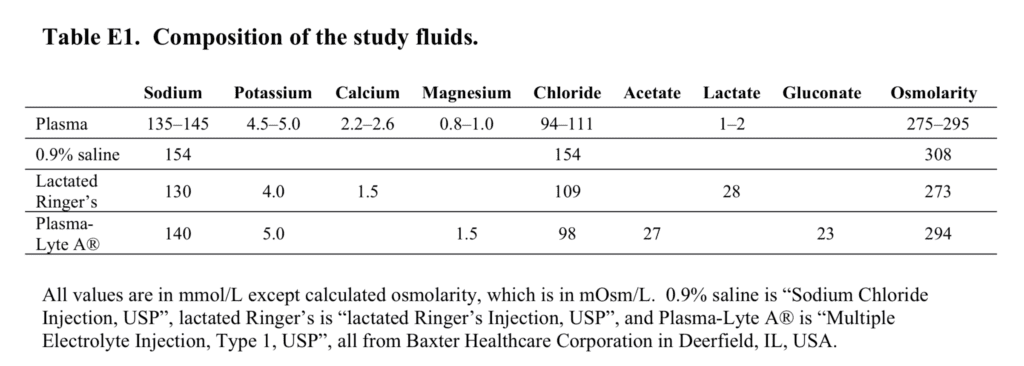
- Composition of Study Fluids
Author Conclusion: “Among patients with sepsis in a large randomized trial, use of balanced crystalloids was associated with a lower 30-day in-hospital mortality compared to use of saline.”
Clinical Take Home Point: We have stated before on the blog that it makes more physiological sense to use balanced crystalloids in large volume (>2L) resuscitation, and given the weight of evidence if you are using NS or a balanced crystalloid as your resuscitation fluid of choice keep using your fluid of choice. It appears now in this secondary analysis of the SMART trial there appears to be a lower 30d in-hospital mortality with the use of lactated Ringer’s solution vs saline which pushes the needle even further to support use of balanced crystalloids over saline in patients with sepsis.
References:
- Brown RM et al. Balanced Crystalloids Versus Saline in Sepsis: A Secondary Analysis of the SMART Trial. Am J Respir Crit Care Med 2019. PMID: 31454263
- Semler MW et al. Balanced Crystalloids Versus Saline in Critically Ill Adults. NEJM 2018. PMID: 29485925
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post SMART Trial Part 2: Secondary Analysis of Balanced Crystalloids vs Saline in Sepsis appeared first on REBEL EM - Emergency Medicine Blog.