
 Background: Previous evidence has found that oseltamivir reduces median time to alleviation of influenza symptoms over placebo by about 1 day. The benefits were greater when treatment was initiated within 24 to 48 hours of symptoms onset. Many previous trials have been criticized for under-recruiting, selective reporting of outcomes, and not including enough children/older patients. Additionally, use of oseltamivir is known to cause side effects such as headaches, nausea/vomiting and, in some cases, neuropsychiatric illness. The CDC currently recommends treatment with oseltamivir in patients with confirmed or suspected influenza who are hospitalized, severely ill, or have higher risk for influenza complications as well as consideration of treatment for symptomatic outpatients if treatment can be initiated within 48 hours.
Background: Previous evidence has found that oseltamivir reduces median time to alleviation of influenza symptoms over placebo by about 1 day. The benefits were greater when treatment was initiated within 24 to 48 hours of symptoms onset. Many previous trials have been criticized for under-recruiting, selective reporting of outcomes, and not including enough children/older patients. Additionally, use of oseltamivir is known to cause side effects such as headaches, nausea/vomiting and, in some cases, neuropsychiatric illness. The CDC currently recommends treatment with oseltamivir in patients with confirmed or suspected influenza who are hospitalized, severely ill, or have higher risk for influenza complications as well as consideration of treatment for symptomatic outpatients if treatment can be initiated within 48 hours.
Paper: Butler CC et al. Oseltamivir Plus Usual Care Versus Usual Care for Influenza-Like Illness in Primary Care: An Open-Label, Pragmatic Randomised Controlled Trial. Lancet 2020. PMID: 31839279
Clinical Question: Does adding antiviral treatment to usual primary care for patients with influenza-like illness reduce the time to recovery?
What They Did:
- Open-label, pragmatic, adaptive, randomized controlled trial
- 15 European countries during three seasonal influenza seasons
- Patients presenting with influenza like illness to primary care randomized to:
- Oseltamivir 75mg PO BID x5d + usual care
- For children <13 years oseltamivir given in oral suspension according to weight:
- 10 – 15kg: 30mg
- >15 -23kg: 45mg
- >23 – 40kg: 60mg
- >40kg: 75mg
- Usual care alone
- For children <13 years oseltamivir given in oral suspension according to weight:
- Trial designed and powered to assess oseltamivir benefit overall and in 36 prespecified subgroups defined by age, comorbidity, previous symptom duration, and symptom severity
- Patients stratified by:
- Age: <12, 12 – <65, and ≥65 years
- Overall severity of influenza-like illness: mild, moderate, or severe)
- Relevant comorbidity: yes or no for heart disease, diabetes, chronic respiratory condition, hepatic, hematological, neurological or neurodevelopmental condition, stroke or TIA, or overnight hospital stay in previous year
- Duration of symptoms since onset: ≤48hrs or >48 to 72hrs
- Oseltamivir 75mg PO BID x5d + usual care
Outcomes:
-
Primary: Time to recovery as recorded on a daily journal
- Defined as return to usual activities, with fever, headache, and muscle aches minor or absent
-
Secondary:
- Cost-effectiveness of adding antiviral treatment to usual primary care
- Incidence of hospital admissions
- Complications related to influenza-like illness
- Repeat attendance in general practice
- Time to alleviation of symptoms of influenza-like illness
- Incidence of new or worsening symptoms
- Time to initial reduction in severity of symptoms
- Use of additional symptomatic and prescribed medication
- Transmission of infection within household
Inclusion:
- Age ≥1 year
- Symptoms of influenza-like illness
- Defined as: sudden onset of self-reported fever, with at least:
- One respiratory symptom (cough, sore throat, or runny/congested nose)
- One systemic symptom (headache, muscle ache, swets/chils, or tiredness)
- With symptom duration of ≤72hrs during a seasonal influenza epidemic
- Defined as: sudden onset of self-reported fever, with at least:
Exclusion:
- Chronic renal failure
- Substantially impaired immunity (i.e. long-term oral steroids, chemotherapy, or immune disorder)
- Patients who should be prescribed immediate antiviral treatment or immediate hospitalization in the opinion of the responsible clinician
- Allergy to oseltamivir
- Scheduled for elective surgery or other procedures requiring general anesthesia during the subsequent 2 weeks
- Life expectancy estimate of <6 months
- Severe hepatic impairment
- Unable to be randomized within 72 hours after onset of symptoms
- Requirement for any live viral vaccine in the next 7 days
- Pregnant/lactating/breast feeding women
Results:
- 3266 participants recruited
- 3059 participants included for the primary outcome
- 1590 (52%) participants had confirmed influenza infection
- 2151 (66%) participants randomized within 48hrs of symptom onset
- Flu vaccination present in 10% of the population
- 96% of participants initiated oseltamivir treatment and 80% reported completing the full course
- Overall people returned to usual activities with mild residual symptoms minimally interfering after about 6.5 days.
-
Overall time to recovery shorter with oseltamivir (Primary Outcome):
- 1.02 days (95% [BCrI] 0.74 to 1.31)
- HR 1.29; 95% Bayesian credible interval (BCrI) 0.74 to 1.31
- Estimated mean benefit from oseltamivir:
- Pts <12 years: 0.70 (95% [BCrI] 0.30 to 1.20)
- Pts ≥65 years: 3.20 (95% [BCrI] 1.00 to 5.50)
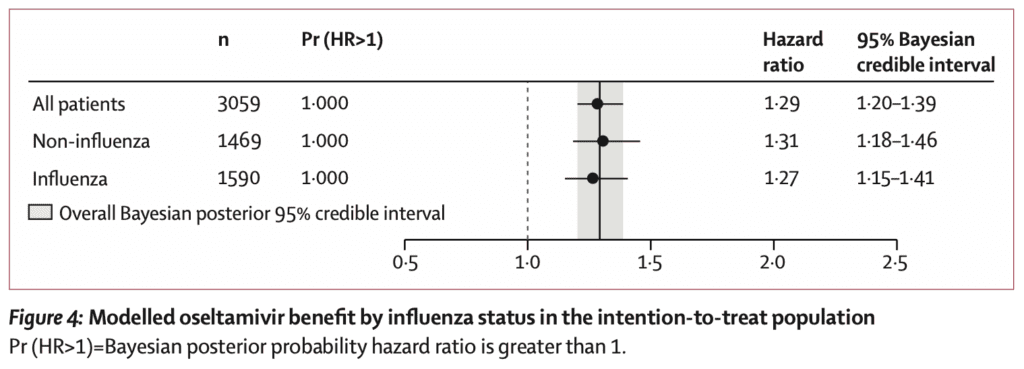
- Estimated mean benefit from oseltamivir in influenza vs non-influenza diagnosis was similar regardless of influenza status
- No differences identified in patient-reported repeat visits with health-care services, hospitalizations, or x-ray confirmed pneumonia
- Reports of new infections within the household:
- Oseltamivir + Usual Care: 39%
- Usual Care Alone: 45%
- Absolute Difference 6.0%; 95% CI 2.1 to 10.0
- Incidence of new or worsening nausea/vomiting:
- Oseltamivir + Usual Care: 21.0%
- Usual Care Alone: 16.0%
Strengths:
- Important to note that the funder of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report
- Groups were very well balanced in terms of age, comorbidity, severity of influenza-like illness, and symptom duration
Limitations:
- This was an open-label study, with no placebo used and drugs not masked. This has huge implications in bias of a subjective outcome
- Non-consecutive recruitment. Only 5500 patients over 3 years of influenza. This creates a selection bias
- Standard care was not standardized. We don’t know anything about other medications used as far as how often, and what doses
- More antibiotics used in the usual care group
- Clinicians assessment of severity of influenza-like illness as mild, moderate, or severe was not well defined (i.e. subjective)
- ≈5500 patients were eligible, however 2235 were excluded with nearly half not willing or able to comply with the trial. We have no idea what happened to these patients
- Majority of patients were in the 12 to 65-year age group. Only 14% were < 12 years and 6% in the ≥ 65 years.
- Participants only recorded symptoms once a day, therefore smaller intervals of time (i.e. hours) may not have been detected
- The primary outcome is a subjective one (thresholds for returning to work may be different for different people)
- Only 10% of the participants were vaccinated for influenza. There could be a lesser effect seen in a population where vaccination is performed more regularly
Discussion:
- Overall, people returned to usual activities with mild residual symptoms after about 6.5 days. The addition of oseltamivir decreased this by about 1 day overall (which is consistent with previous evidence).
- Older, sicker, patients with comorbid conditions, or longer previous symptom duration could expect to return to usual activities with mild residual symptoms 2 to 3 days earlier with oseltamivir
- The biggest issue in this trial is non-blinding in a study with a self-reported, soft, subjective outcome. Everyone knew what they were taking.
- 10% of population got influenza vaccination. This is an extremely low rate. Looking at the CDC website (Link is HERE), in adults, 18 years of age and older, there was an overall ≈40% vaccination rate in the US (2010 – 2019). Although the flu vaccine is not perfect, it reduces flu illness, hospitalizations, and deaths. In a study with no objective benefits (i.e. hospitalization, pneumonia), it seems focusing on vaccination would be more beneficial than feeling better 1 or 2 days sooner.
- In this trial participants with confirmed influenza did not benefit more than those testing negative
- As the flu swab does not have 100% sensitivity, there may have been some false negatives in the non-confirmed influenza group
- Inconsistent swabbing techniques could have also affected this
- Effectiveness of oseltamivir in the influenza negative patients raises concerns about validity of the outcomes
- Interestingly in this study, oseltamivir started after 48 hours after symptom onset had a similar effect as the patients who started it <48 hours
Author Conclusion: “Primary care patients with influenza-like illness treated with oseltamivir recovered one day sooner on average than those managed by usual care alone. Older, sicker patients with comorbidities and longer previous symptom duration recovered 2 – 3 days sooner.”
Clinical Take Home Point: This study should not change practice. In a non-blinded study with a subjective outcome, we would expect patients who are getting the “fancy pill” to feel better faster. Additionally…
- Oseltamivir works on neuraminidase. This enzyme is not found on other viruses, and there is no biological mechanism for it to have an effect in these patients
- Objective outcomes of hospitalization and x-ray confirmed pneumonia showed no benefit with oseltamivir
- There is an increase in nausea/vomiting in the patients taking oseltamivir
References:
- Butler CC et al. Oseltamivir Plus Usual Care Versus Usual Care for Influenza-Like Illness in Primary Care: An Open-Label, Pragmatic Randomised Controlled Trial. Lancet 2020. PMID: 31839279
For More Thoughts on This Topic Checkout:
- REBEL EM: The Tamiflu Debacle
- REBEL Review 80: Oseltamivir (Tamiflu) for Treatment of Influenza
- First10EM: Tamiflu Doesn’t Work
- The SGEM: SGEM #312 – Oseltamivir is Like Bad Medicine – For Influenza
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post The ALIC4E Trial: Oseltamivir + Usual Care vs Usual Care Alone appeared first on REBEL EM - Emergency Medicine Blog.


