 Background: COVID-19 became an international pandemic in March of 2020, claiming the lives of millions of people worldwide. To combat this deadly virus, an unprecedented global effort led to the development of many new vaccines against COVID-19 within a year. Some of these vaccines were made from a new mRNA programmed technique, others used traditional technology. These advances reduced the transmission of the virus and proved efficacious in preventing hospitalizations, severe disease, and death from COVID-19 and its variants.
Background: COVID-19 became an international pandemic in March of 2020, claiming the lives of millions of people worldwide. To combat this deadly virus, an unprecedented global effort led to the development of many new vaccines against COVID-19 within a year. Some of these vaccines were made from a new mRNA programmed technique, others used traditional technology. These advances reduced the transmission of the virus and proved efficacious in preventing hospitalizations, severe disease, and death from COVID-19 and its variants.
The rise of SARS-Cov2 variants with mutations to the spike protein presents challenges to the immunity conferred by vaccines and immunity from infection. Waning immunity (whether from infection or vaccine) led to recommendations for booster doses. However, it’s unclear whether individuals should be boosted with the same vaccine as their primary series (homologous) or with a different vaccine (heterologous). There may be potential advantages to heterologous boosters, but data is lacking.
Studies from Israel and Qatar showed that immunity conferred from vaccines wanes over time, although this immunity seems to be preserved against severe disease in younger adults. Due to waning immunity, a booster vaccine has been recommended by public health officials. Although booster vaccines have been recommended, no guidelines on booster administration were proposed leaving the question of homologous vs heterologous combination of vaccines and their effectiveness.
Article: Munro AP et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial PMID: 34863358
Clinical Question: Are the various COVID-19 booster vaccine combinations safe, effective as well as provide an immunogenic response to COVID-19 and its variants?
What They Did:
- Multicenter, randomized, controlled phase 2 trial
- Investigators enrolled patients at 18 community and secondary care sites in the United Kingdom.
- Participants were randomly assigned by permuted block randomization, to one of three groups: A, B, or C.
- Randomized to receive one of seven different vaccine boosters including three half-dose formulations or a control.
Population:
Inclusion:
- Adults ≳ 30 years of age,
- Received two doses of either Pfizer or AstraZeneca
-
More than 84 days elapsed since the second dose at the time of enrolment.
- Some sites were permitted to enroll participants who were at least 70 days after a second dose of AstraZeneca vaccine.
Exclusion:
- Participants who are pregnant or planning to become pregnant during the first 3 months following vaccination.
- Administration of immunoglobulins and/or any blood products within three months prior.
- Immunosuppressive or immunodeficient state
- History of allergic reaction to any component of study vaccines
- History of anaphylaxis
- Current diagnosis of or treatment for cancer
- Bleeding disorder
- Continuous use of anticoagulants
- History of cerebral venous sinus thrombosis, antiphospholipid syndrome or heparin-induced thrombocytopenia and thrombosis (HITT or HIT type 2)
- Suspected or known current alcohol or drug dependency
- Any other significant disease which may significantly increase the risk to the volunteer because of participation in the study, affect the ability of the volunteer to participate in the study or impair interpretation of the study data
- Severe and/or uncontrolled cardiovascular disease, respiratory disease, gastrointestinal disease, liver disease, renal disease, endocrine disorder or neurological illness
- History of active or previous autoimmune neurological disorders
- Significant renal or hepatic impairment
- There were 20 exclusion categories. For a full list see appendix 2 page 34 [Link is here]
Intervention:
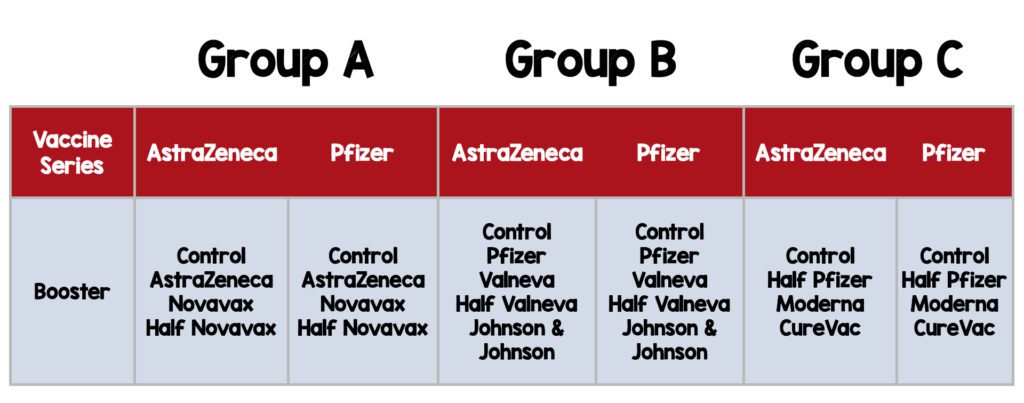
Control:
- Quadrivalent meningococcal conjugate vaccine (MenACWY)
Outcomes:
Coprimary Outcomes:
-
Safety and reactogenicity: as recorded in participant diaries or ascertained at follow up visits.
- Solicited or unsolicited adverse events
- Adverse events of special interest
- Serious adverse events following vaccination
-
Immunogenicity:
- Anti-spike protein IgG at day 28 follow-up
Key Secondary Outcomes: Immunogenicity assays
- Neutralizing antibody titers against wild-type (original strain) COVID-19 strain and pseudovirus neutralization.
- T-cell response against wild-type and SARS-CoV-2 virus variants of concern (alpha, beta, and delta)
- Cellular immune responses against wild-type and SARS-CoV-2 virus variants of concern (alpha, beta, and delta)
Results:
- 3498 participants screened
- 2883 participants recruited for inclusion
- 2878 participants received a third dose vaccine
Primary Outcome Results:
Immunogenicity: Anti-spike protein IgG at day 28 follow-up
-
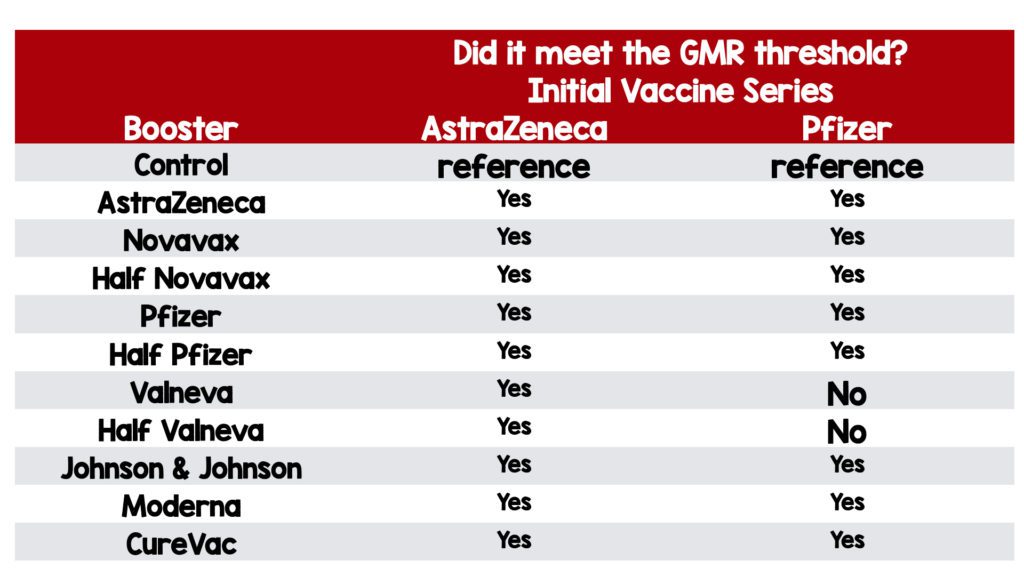
AstraZeneca primed group:
- All vaccines induced significantly higher anti-spike IgG at 28 days post boost, compared with controls.
-
- All study vaccines except AstraZeneca, Valneva, and half Valneva significantly induced cellular responses by T-cell ELISpot.
- GMR ranged from 1.8 (99% CI 1.5–2.3) in the half Valneva group to 32.3 (24.8–42.0) in the Moderna group.
-
Pfizer primed group:
- All vaccines induced significantly higher anti-spike IgG at 28 days post boost, compared with controls.
- Range from 1·3 (99% CI 1·0–1·5) in the half Valneva group to 11·5 (9·4–14·1) in the Moderna group.
- The upper limit of the 99% CI for Valneva and half Valneva did not reach the pre-established minimum clinically important difference of 1·75.
Safety and Reactogenicity:
-
Any Reaction:
- In the group primed with the AstraZeneca vaccine, mRNA vaccines or Johnson & Johnson showed increased systemic and local adverse events.
- In the group primed with the Pfizer vaccine, patients boosted with Moderna, CureVac, AstraZeneca, and Johnson & Johnson reported more frequent local and systemic reactions
- Pain at the injection site was the most common local reaction within 7 days after the vaccination.
- Fatigue and headache were the most common systemic reactions within 7 days after all vaccines.
- Reactogenicity was greater in the 30–69 aged group regardless of the vaccine received.
- Moderna was the most reactogenic mRNA vaccine.
-
Severe Local and Systemic Reactions:
- AstraZeneca primed group: 11.6% boosted with Moderna reported severe fatigue.
- Pfizer primed group: ≈ 5% boosted with AstraZeneca, Moderna, CureVac, and Johnson & Johnson reported malaise.
-
Adverse Events
- 1306 adverse events reported from 912 participants
- 6 events possibly related to study vaccine
- 21 participants reported a PCR test result positive for SARS-CoV-2 without requiring hospitalization
- 24 serious adverse events where most were deemed to be unlikely due to the vaccine or not related to the vaccine
Secondary Outcome Results:
Neutralizing Antibodies:
-
AstraZeneca primed group:
- GMRs for pseudotype virus-neutralizing antibodies against wild-type consistent with anti-spike IgG
-
Pfizer primed group:
- GMRs for pseudotype virus-neutralizing antibodies against wild-type consistent with anti-spike IgG
- Pseudoneutralizing antibodies (NT50) were reduced for delta variant, relative to wild-type, across all vaccines after AstraZeneca and Pfizer.
- No vaccine showed better cross-protective immunity than others (ie, similar GMRs were observed for delta and wild-type for all study vaccines, when comparing with control groups)
T-Cell Response:
-
AstraZeneca primed group:
- All study vaccines except AstraZeneca, Valneva, and half Valneva significantly induced T-cell responses
-
Pfizer primed group:
- The T-cell-boosting effects of Novavax and half Novavax were lower in people who had received Pfizer compared with those who primed with AstraZeneca.
- The geometric mean of T-cell responses in the half Novavax group was not significantly higher than control (1·4, 95% CI 0·89–2·2).
- All other vaccines showed higher cellular responses compared with controls.
- T-cell responses against delta and beta were similar to wild-type
- All other vaccines showed higher cellular responses compared to control.
Strengths:
- Asks a clinically relevant question of critical importance such as vaccines safety
- Blinded, Multicenter, RCT increases study generalizability and limits bias
-
Blinding procedures are sound
- Participants, laboratory staff and clinical study team were blind to treatment allocation
- Randomization was concealed, vaccines prepared out of sight, masking tape was applied to vaccine syringes to conceal dose, volume, and appearance
- Performed an intention-to-treat analysis on immunogenicity outcomes.
Limitations:
- Some outcomes were disease-oriented and not patient-oriented.
- Study was performed in a single country (UK) which limits generalizability.
- Not all vaccines studied are widely available in all regions
- Study participants represent a largely homogeneous group, 95.4% were white. This limits generalizability to a more diverse population.
-
There were differences in baseline characteristics as some groups had more comorbid conditions than others.
- Investigators attributed this to differences in availability—Pfizer was released first.
- AND elderly patients and patients with comorbid conditions were prioritized.
- Excludes subjects <30 years old which limits generalizability.
- Generally, healthy cohort limits generalizability to a broader cohort
- Study took place prior to the Omicron outbreak potentially limiting generalizability to current and future variants.
- Lots of exclusion criteria ultimately limiting generalizability to high-risk patient populations.
- The threshold of minimal clinical difference of GMRs was 1.75 and the significance of this value is unclear as well as it is unclear exactly how or why this value was chosen
-
Lots of important information was only available in the appendix
- Exclusion criteria
- List of secondary outcomes
- The definitions of adverse events, and adverse events of special interest are unclear.
Discussion:
GMC (Geometric Mean Concentration) and GMR (Geometric Mean Ratio):
- GMC: The average concentration of anti-spike IgG in each group.
- GMR: A mathematical expression of the relative increase in concentration of anti-spike IgG, in patients receiving one of the experimental boosters vs those receiving the control.
- GMC and GMR are likely abstract and foreign to anyone except for an Immunologist perhaps.
- The investigators decided that the minimum clinically important difference for GMC would be 1.75. This value seems somewhat subjective and arbitrary. Investigators don’t reveal how or why this number was chosen.
- Interpretation of the paper would lead you to believe that higher values of GMC and GMR are better. However, these are disease (or in this case vaccine) oriented outcomes. It’s unclear from the paper whether higher GMCs and GMRs confer more protection from infection.
Safety:
- All the vaccines in this study have shown that they have minimal side effects with local and systemic reactions similar between all vaccines most commonly pain and headache, fatigue
- Severe reactions were reported as less than 5% across all vaccines except fatigue and malaise
- These are important findings in our current society with many skeptical and worried about vaccine safety.
Immunogenicity:
- For the AstraZeneca primed group, all vaccines induced significantly higher anti-spike IgG at 28 days post booster.
- For the Pfizer group, all vaccines induced significantly higher anti-spike IgG at 28 days post booster except for Valneva and half Valneva.
- All of the vaccines with the exception of Valneva following Pfizer were superior to the control whether a homologous or heterologous strategy was used.
- There was acceptable reactogenicity, all vaccines showed good correlation between pseudoneutralizing assay against, wild type and delta
- Vaccines appear to neutralize wild type and delta effectively but to a lesser effect with the variant.
- Although these studies showed good immunological response to almost all of the vaccines combinations, these results are not patient-centered outcomes.
- It is unclear from this paper whether the degree of immunologic response will directly correlate to vaccine efficiency.
Homogeneous Group
- Elderly patients with comorbid conditions are particularly vulnerable to SARS-CoV19. Investigators managed enrollment so that approximately half the participants were above the age of 75 years of age.
- However, the cohort of patients was predominantly white.
- Additionally, investigators excluded many potential patients with chronic medical conditions.
- So, minorities and patients with chronic medical conditions are vastly underrepresented in this study.
Authors Conclusion: “This trial has demonstrated the potential of all vaccines tested (ChAd, BNT, m1273, NVX, Ad26, CVn, and VLA) to boost immunity following an initial course of ChAd/ChAd and of six vaccines (ChAd, BNT, m1273, NVX, Ad26, and CVn) following an initial course of BNT/BNT. All vaccines showed acceptable side-effect profiles, although some schedules were more reactogenic than others.”
Clinical Bottom Line:
We agree with the author’s conclusions. All vaccine regimens were safe and adverse events were rare and relatively minor. Nearly all adverse reactions were unrelated to vaccination. Nearly all of the vaccines boosted immunity and were superior to the control regardless of a heterologous or homologous vaccine schedule.
References:
- Munro AP et al. Safety and immunogenicity of seven COVID-19 vaccines as a third dose (booster) following two doses of ChAdOx1 nCov-19 or BNT162b2 in the UK (COV-BOOST): a blinded, multicentre, randomised, controlled, phase 2 trial PMID: 34863358
- Levin EG, Lustig Y, Cohen C, et al. Waning Immune Humoral Response to BNT162b2 Covid-19 Vaccine over 6 Months. N Engl J Med. 2021;385(24):e84. PMID: 34614326
- Chemaitelly H, Tang P, Hasan MR, et al. Waning of BNT162b2 Vaccine Protection against SARS-CoV-2 Infection in Qatar. N Engl J Med. 2021;385(24):e83. PMID: 34614327
- Tenforde MW, Self WH, Naioti EA, et al. Sustained Effectiveness of Pfizer-BioNTech and Moderna Vaccines Against COVID-19 Associated Hospitalizations Among Adults – United States, March-July 2021. MMWR Morb Mortal Wkly Rep. 2021;70(34):1156-1162. Published 2021 Aug 27. PMID: 34437524
Guest Post By:

Allana Rodriguez, MD
PGY-3, Emergency Medicine Resident
Saint Joseph’s University Medical Center, Paterson New Jersey
Email: sighs369@gmail.com

Marco Propersi, DO FAAEM
Assistant Professor, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson New Jersey
Twitter: @marco_propersi
Steven Hochman, MD FACEP
Associate Professor, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson New Jersey
Twitter: @hochmast
Post-Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The COV-BOOST Trial: Safety and Immunogenicity of Boosters appeared first on REBEL EM - Emergency Medicine Blog.

