
 Background: Despite continued debate on the efficacy of alteplase (tPA), it currently remains one of the major interventions directed at patients presenting with acute ischemic stroke. The current standard dose of the drug is 0.9 mg/kg given over 1 hour. It is unclear whether lower doses would be equally effective in increasing good neurologic outcomes after stroke while simultaneously decreasing the rate of intracerebral hemorrhage (ICH); the most serious side effect. Evidence showing that lower doses of tPA are non-inferior to standard-dose tPA could lead to a shift in treatment.
Background: Despite continued debate on the efficacy of alteplase (tPA), it currently remains one of the major interventions directed at patients presenting with acute ischemic stroke. The current standard dose of the drug is 0.9 mg/kg given over 1 hour. It is unclear whether lower doses would be equally effective in increasing good neurologic outcomes after stroke while simultaneously decreasing the rate of intracerebral hemorrhage (ICH); the most serious side effect. Evidence showing that lower doses of tPA are non-inferior to standard-dose tPA could lead to a shift in treatment.
Clinical Question:
Is low-dose tPA non-inferior to standard-dose tPA in terms of death or disability at 90 days in the treatment of acute ischemic stroke presenting within 4.5 hours of symptom onset?
Article:
Anderson CS et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. NEJM 2016. PMID: 27161018
PICO:
- Population: Patients > 18 years of age with a clinical diagnosis of acute ischemic stroke who met guideline recommended criteria for treatment with intravenous tPA within 4.5 hours of symptom onset. Patients had to be independent at baseline (mRS < 1).
- Intervention: Low-dose (0.6 mg/kg) tPA (administered as 15% of dose as IV bolus followed by 85% of dose as infusion over 1 hour)
- Control: Standard-dose (0.9 mg/kg) tPA (administered as 10% of dose as IV bolus followed by 90% of dose as infusion over 1 hour)
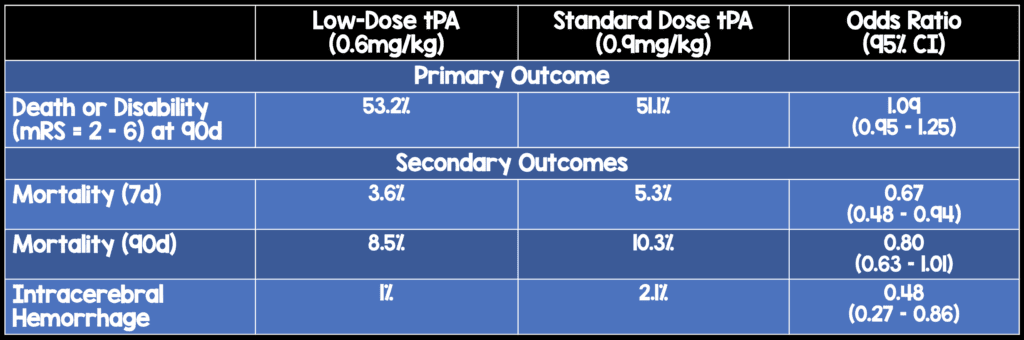
- Outcome (primary): Death or disability (mRS = 2-6) at 90 days
- Outcomes (secondary): ICH
Design:
- Multicenter, prospective, randomized, open-label trial
Excluded:
- Unlikely to benefit from the therapy due to pre-existing disability
- Another medical illness interfered with the outcome assessment
- Unlikely to adhere to follow up
- Unable to consent
- Previously enrolled in the ENCHANTED study
Results:
- 3310 patients recruited and 3206 patients analyzed from 111 centers in 13 countries
- Non-inferiority prespecified as an upper limit for non-inferiority of 1.14. This noninferiority margin was derived from a Cochrane meta-analyses of alteplase
Strengths:
- Large study asking a clinically relevant question with important patient centered outcomes being considered
- Assessors of outcomes at follow up were blinded to treatment allocation
- Loss to follow up was minimal in both arms (low dose: 47, standard dose: 44)
Limitations:
- Open label study design opens up trial to numerous biases
- Co-primary endpoint (death or disability)
- It is unclear whether patients were recruited consecutively or not
- > 60% of patients were of Asian descent which may limit external validity
- Follow-up at 28 and 90-days done by in-person and telephone interview. Heterogeneity of assessment approach may lead to imprecise results
- The authors of the study had significant conflicts of interest
Author’s Conclusions:
“This trial involving predominantly Asian patients with acute ischemic stroke did not show the noninferiority of low-dose alteplase to standard-dose alteplase with respect to death and disability at 90 days. There were significantly fewer symptomatic intracerebral hemorrhages with low-dose alteplase.”
Our Conclusions:
Low-dose tPA did not meet the upper limit of the prespecified non-inferiority threshold for the odds ratio in comparison to standard-dose tPA for the primary outcome of death or disability at 90 days. However, low-dose tPA performed extremely well in this study. Low-dose tPA patients were more likely to be alive at both 7 and 90 days with a lower ICH rate.
Potential Impact to Current Practice: As this study did not show non-inferiority of the low-dose tPA approach, we do not think it will alter overall treatment strategies. However, in patients with CVA who are eligible for systemic thrombolytics and in whom the doctor and patient both think would benefit from the drug but have increased risk of bleeding, low-dose tPA may be provide an alternative approach.
Bottom Line: Low-dose tPA achieved similar outcomes to standard-dose tPA with lower mortality and ICH rates. Although this study does not prove non-inferiority of low-dose tPA, it also does not show superiority of standard-dose tPA.
Check Out More on This Topic Below:
- Rory Spiegel at EM Nerd: The Case of the Non-Inferior Inferiority Continues
- Adrian Wong at The Bottom Line: Low-Dose vs Standard-Dose Alteplase in Acute Ischemic Stroke
- Ryan Radecki at EMLit of Note: ENCHANTED – Positive or Negative?
References:
- Anderson CS et al. Low-dose versus standard-dose intravenous alteplase in acute ischemic stroke. NEJM 2016. PMID: 27161018
- Wardlaw JM et al. Thrombolysis for Acute Ischaemic Stroke. Cochrane Database Syst Rev 2014. PMID: 25072528
Post Peer Reviewed By: Salim Rezaie, MD (Twitter: @srrezaie)
The post The ENCHANTED Trial: Is Low-Dose the Right Dose for Intravenous tPA in Acute Ischemic Stroke? appeared first on REBEL EM - Emergency Medicine Blog.
