
 Background: Bronchiolitis is an acute inflammatory injury of the distal smaller airways, most commonly caused by viral infections. There have been a host of medications studied in the treatment of bronchiolitis, including steroids, albuterol, epinephrine, and inhaled hypertonic saline, with none proving to be effective in treatment. Oxygen therapy via high-flow nasal cannula (HFNC) as opposed to standard nasal prongs provides some positive airway pressure which decreases work of breathing, improves oxygenation, and rates of intubation. The trial we are going to review today (The PARIS Trial) evaluated early high-flow oxygen therapy vs standard oxygen therapy in infants with bronchiolitis and hypoxemia in both the emergency department and general pediatric ward settings.
Background: Bronchiolitis is an acute inflammatory injury of the distal smaller airways, most commonly caused by viral infections. There have been a host of medications studied in the treatment of bronchiolitis, including steroids, albuterol, epinephrine, and inhaled hypertonic saline, with none proving to be effective in treatment. Oxygen therapy via high-flow nasal cannula (HFNC) as opposed to standard nasal prongs provides some positive airway pressure which decreases work of breathing, improves oxygenation, and rates of intubation. The trial we are going to review today (The PARIS Trial) evaluated early high-flow oxygen therapy vs standard oxygen therapy in infants with bronchiolitis and hypoxemia in both the emergency department and general pediatric ward settings.
What They Did:
- Paediatric Acute Respiratory Intervention Study (PARIS)
- Multicenter, randomized, controlled trial
- Infants <12 months of age with bronchiolitis and need for supplemental oxygen therapy
- High-flow oxygen therapy (High-flow group) vs standard oxygen therapy (Standard therapy group)
- Infants in standard therapy group could receive rescue high-flow O2 if their condition met criteria for treatment failure
Treatments:
- High Flow Oxygen Therapy:
- Heated & humidified high-flow oxygen at a rate of 2L/kg/min via Optiflow system
- Fraction of inspired oxygen (FiO2) adjusted to obtain O2 sat levels between 92 – 98% or 94 – 98% at some of the treatment hospitals as they had a higher minimum oxygen threshold
- Standard Therapy Oxygen:
- Supplemental oxygen through nasal cannula up to 2L/min
- Maintain oxygen saturation level between 92 – 98% or 94 – 98% at some of the treatment hospitals as they had a higher minimum oxygen threshold
Outcomes:
- Primary: Escalation of care due to treatment failure (defined as meeting ≥3 out of 4 clinical criteria
- Persistent tachycardia
- Persistent tachypnea
- Hypoxemia
- Medical review triggered by a hospital early-warning tool
- Secondary:
- Duration of hospital stay
- Duration of oxygen therapy
- Rates of transfer to a tertiary hospital
- Rate of ICU admission
- Rate of intubation
- Rate of adverse events
Inclusion:
- Infants <12 months of age
- Clinical signs of bronchiolitis
- Need for supplemental oxygen therapy to keep oxygen saturation between 92 – 98% (or 94 – 98% at some hospitals who had higher saturation thresholds)
Exclusion:
- Critically ill infants who had immediate need for respiratory support and ICU admission
- Cyanotic heart disease
- Basal skull fracture
- Upper airway obstruction
- Craniofacial malformation
- Receiving oxygen therapy at home
Results:
- 1472 infants
- Escalation of Care:
- High-Flow Group: 12%
- Standard Therapy Group: 23%
- P <0.001
- NNT = 9
- Fragility Index = 51
- No difference in:
- Duration of hospital stay
- Duration of oxygen therapy
- Adverse Events: 1 infant in each group suffered a pneumothorax (<1% of infants) – no drainage needed in either case
- 167 infants who had treatment failure with standard therapy
- 102 (61%) had a response to high flow oxygen therapy
Strengths:
- Protocol published previous to enrollment and completion of trial
- Clinically important primary endpoint particularly in hospitals without pediatric ICUs
- Investigators were unaware of trial outcome until the trial was completed
- High-flow equipment for trial was donated by Fisher and Paykel Healthcare, who had no involvement in the design, analysis of data, or preparation of manuscript
- Pre-specified subgroups were evaluated for heterogeneity of the studies results audemars piguet replica
Limitations:
- Unblinded study – masking of the assigned treatment was not possible due to visually obvious differences between the two interventions. This may minimize the validity of the results of this paper due to placebo effect/bias or effect on physician decisions.
- Clinicians could escalate therapy if they were concerned for other clinical reasons, which is a subjective decision – this occurred in 34% of the patients who did not meet 3 out of the 4 criteria for escalation of care
- The respiratory rate was quite a bit higher in the HFNC group vs Standard therapy group (62.6 +/- 15.2 vs 54.6 +/ 12.4), which could potentially mean that the HFNC group was a bit sicker and therefore the results of this study would underestimate the true benefit of HFNC vs Standard therapy
Discussion:
- Respiratory Syncytial Virus (RSV) was the most common virus detected in this study at approximately 55% of cases
- Approximately 18% of the included patient population had premature (<37weeks) birth
- Non-study treatment and medications received:
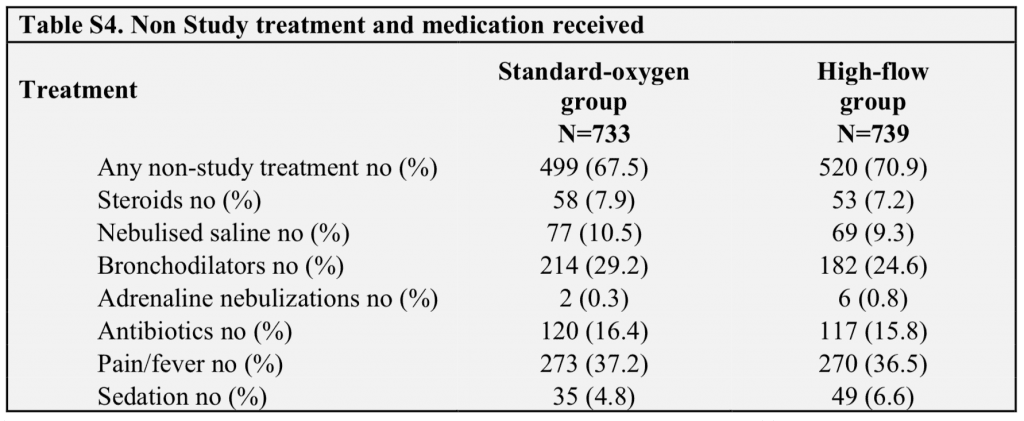
- Escalation rates were quite higher in the peds hospital with an ICU. The escalation rate was also closer between HFNC and standard O2 groups in hospitals with an ICU as well. It is possible, that HFNC may be of greatest advantage at places that would have to transfer for escalation of care. Interestingly at the sites that did not have an ICU, the treatment failure rate was significantly lower in the HFNC group vs standard therapy group: 7% vs 28% (NNT = 5) compared to sites that did have an ICU 14% vs 20% (NNT = 17)
Author Conclusion: “Among infants with bronchiolitis who were treated outside an ICU, those who received high-flow oxygen therapy had significantly lower rates of escalation of care due to treatment failure than those in the group that received standard oxygen therapy.”
Clinical Take Home Point: This is the largest trial to date evaluating the effectiveness of HFNC in bronchiolitis in infants <12months of age, hypoxemia, and either in the ED or pediatric ward. Based on this well done study it appears that HFNC is superior to standard oxygen therapy with a NNT = 9.
References:
- Franklin D et al. A Randomized Trial of High-Flow Oxygen Therapy in Infants with Bronchiolitis. NEJM 2018. PMID: 29562151
For More Thoughts on This Topic Checkout:
- Ben Lawton at Don’t Forget the Bubbles: Paris in the Autumn
- Fraser Magee at The Bottom Line: A Randomised Trial of High-Flow Oxygen Therapy in Infants with Bronchiolitis
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)
The post The PARIS Trial: HFNC in Infants with Bronchiolitis appeared first on REBEL EM - Emergency Medicine Blog.
