
 Background: The optimal approach to risk stratifying patients for pulmonary embolism (PE) remains elusive. Multiple decision instruments are available with varying degrees of complexity and acceptance among emergency medicine clinicians (Wells, Geneva, PERC, YEARS, age-adjusted D-dimer).
Background: The optimal approach to risk stratifying patients for pulmonary embolism (PE) remains elusive. Multiple decision instruments are available with varying degrees of complexity and acceptance among emergency medicine clinicians (Wells, Geneva, PERC, YEARS, age-adjusted D-dimer).
The 2018 ACEP clinical policy on acute venous thromboembolic (VTE) disease [Reviewed here] affirms the use of the PERC algorithm [Reviewed here] in low-risk patients and age-adjusted D-dimer [Reviewed here] in low-intermediate risk patients. In addition, the YEARS score [Reviewed here], developed in 2017, has gained popularity due to its simplicity compared to other complex decision instruments. It has been subsequently externally validated in prospective trials.
Many clinicians integrate PERC and YEARS when evaluating patients with suspected PE. Although this approach makes sense, it is yet to be validated.
Paper: Freund Y et al. Effect of a Diagnostic Strategy Using an Elevated and Age-Adjusted D-Dimer Threshold on Thromboembolic Events in Emergency Department Patients With Suspected Pulmonary Embolism: A Randomized Clinical Trial. JAMA. 2021;326(21):2141-2149. [PMID: 34874418]
Clinical Question: Among PERC-positive emergency department (ED) patients with suspicion for pulmonary embolism (PE), does the combination of the YEARS score and age-adjusted D-dimer threshold safely exclude the diagnosis of venous thromboembolism?
What They Did:
- Cluster-randomized, crossover, noninferiority trial
- 24 EDs approached, 18 EDs participated, 16 in France and 2 in Spain
- Patient enrolled from October 1, 2019, to October 1, 2020
- Each ED was randomized 1:1 to the control strategy for four months, followed, after a 2-month washout period, by the intervention strategy for four months, or vice versa.
-
Randomization was stratified by country and ED size.
- Small vs. large was defined as <50 000 or ≥50 000 patients per year.
- ED physicians enrolled patients.
- The trial was not blinded because of the distinct differences in diagnostic pathways.
Population:
Inclusion Criteria:
- Clinical suspicion of a PE (e.g., acute onset of chest pain, worsening acute dyspnea, or syncope)
- AND Low subjective probability (<15%) with 1 or more PERC score elements
- OR Intermediate subjective probability (16%-50%) of PE.
Exclusion Criteria:
- Patients with a high subjective probability of PE (>50%, i.e., those who should undergo chest imaging without further workup)
- Patients with a low subjective probability of PE with a PERC score of zero (i.e., those for whom PE is ruled out without a D-dimer)
- Severe illness (respiratory distress, hypotension, peripheral oxygen saturation <90%)
- Current anticoagulant treatment
- Current diagnosis of thromboembolism
- Pregnancy
- Correctional facility inmate
- Symptoms related to a cause other than PE
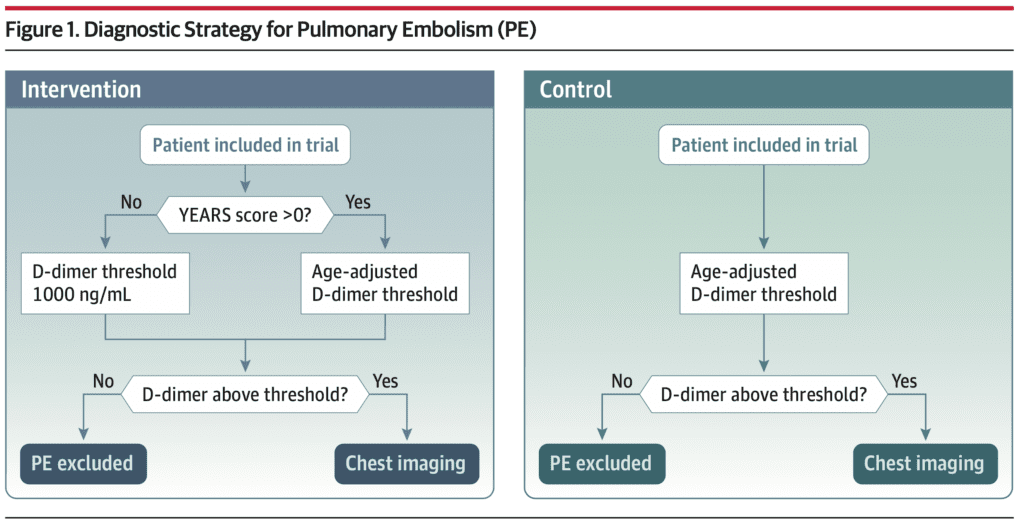
Intervention:
-
PE was considered ruled out if:
- Patients with no YEARS criteria: D-dimer level below 1000 ng/mL FEU
- Patients with > 1 YEARS criteria: D-dimer level below the age-adjusted threshold (age × 10 ng/mL FEU in patients aged ≥50 years)
- Chest imaging (triggered by an elevated D-dimer) was negative
Control:
-
PE was considered ruled out if:
- D-dimer testing with the level below the threshold set at the age-adjusted level
- Chest imaging (triggered by an elevated D-dimer) was negative
Outcome:
Primary Outcome:
- Failure of the diagnostic strategy: Venous thromboembolism (VTE) diagnosis at 3 months after exclusion of PE during the initial ED visit.
-
VTE was defined as:
- Deep vein thrombosis confirmed by venous Doppler ultrasonography
- Intraluminal defect on CTPA
- V/Q mismatch that had a high probability of being caused by a PE
Secondary Outcomes:
- Chest imaging (CTPA or V/Q scan) ordered by ED physicians
- ED length of stay
- Hospital admission following the ED visit
- Anticoagulant administration
- All-cause mortality
- All-cause readmissions at 3 months
-
Two prespecified secondary end-points were not included in this analysis:
- Safety of the 4PEPS score
- Cost-effectiveness of the intervention will be reported in an ancillary analysis.
Results:
-
1414 patients enrolled
- 726 in the intervention strategy group
- 688 in the control strategy group
-
All 1414 patients were included in as-randomized (Intention-to-treat) group.
- 37 patients had a missing primary end-point
- 67 patients deemed ineligible
- 39 patients with major protocol violations
-
1271 patients were included in the per-protocol analysis
- 648 in the intervention strategy group
- 623 in the control strategy group
-
PE was diagnosed in 100 patients during the study period.
- 54 (7.4%) in the intervention group
- 46 (6.7%) in the control group
- Difference: −0.8% [95% CI, −2.0% to 3.5%]
- A total of 9 VTEs at three months were adjudicated in the as-randomized population, including 5 unexpected deaths with no other identified cause.
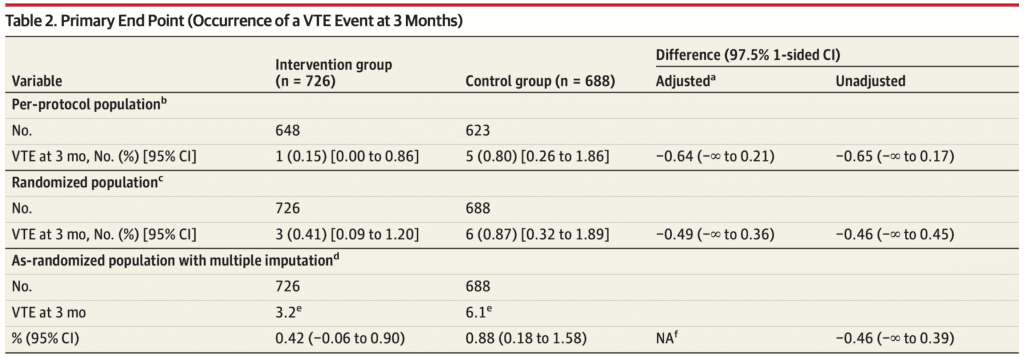
Primary Outcome:
-
In the per-protocol population, 6 PEs were diagnosed at 3 months
- 1 PE in the intervention group
- 5 PE in the control group
-
The failure rate was:
- 0.15% (95% CI, 0.00% to 0.86%) in the intervention
- 0.80% (95% CI, 0.26% to 1.86%) in the control groups
-
Adjusted difference of the failure rate between the 2 groups:
- −0.64% (1-sided 97.5% CI, −∞ to 0.21%)
- Less than the noninferiority margin of 1.35%.
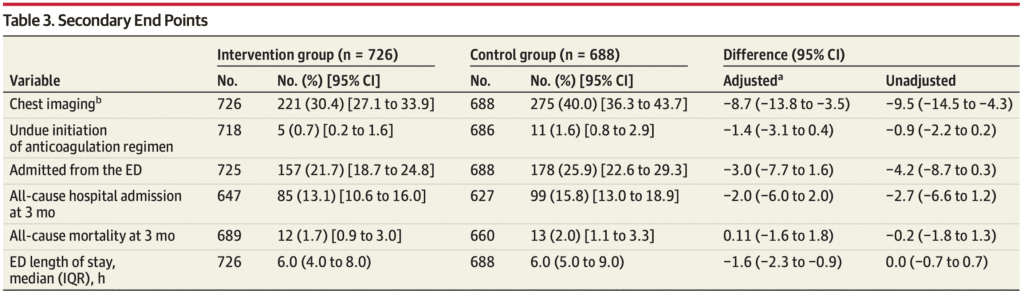
Secondary Outcomes:
- 2 of 6 secondary outcomes reached a statistically significant difference in the intervention group compared with the control group.
- Findings were similar in both as-randomized (intention-to-treat [ITT]) and per-protocol analysis.
-
Chest imaging was performed in the ED in 496 patients (35.1%):
- 221 (30.4%) in the intervention group
- 275 (40.0%) in the control group
- Statistically Significant: Difference, −9.6%; adjusted difference, −8.7% [95% CI, −13.8% to −3.5%]
-
The median ED length of stay:
- 6.0 hours (IQR, 4.0-8.0) in the intervention group
- 6.0 hours (IQR, 5.0-9.0) in the control group
- Statistically Significant: adjusted difference, −1.6 hours [95% CI, −2.4 to −0.9]
Post Hoc Analysis
-
Evaluation of subset of patients who had a change in their diagnostic strategy
- In patients with a YEARS score of one, age-adjusted D-dimer was used, which was similar to the control group.
-
In the per-protocol population, there was a total of 956 patients with a YEARS score of zero.
- 515 in the intervention group
- 441 in the control group
-
No missed PEs in the intervention group
- Failure rate, 0.00% [95% CI, 0.00% to 0.71%],
- Less than the noninferiority margin
-
3 missed PEs in the control group
- Failure rate, 0.68% [95% CI, 0.00% to 1.45%]
-
Chest imaging performed
- 22.9% of patients in the intervention group
- 37.2% in the control group
- Absolute reduction of 14.3% [95% CI, 8.3% to 20.2%]
Strengths:
- Asks a clinically important patient-centered question.
- Outcomes were patient-oriented.
- Multicenter study increased external validity to a more diverse patient population.
- Cluster-randomized, crossover trial limits the potential for bias when blinding is unachievable.
- Pragmatic ED-based trial increases the applicability.
- Computer-generated randomization stratified by country and ED size.
- Appropriate use of noninferiority trial with potential to decrease exposure to radiation.
- The noninferiority threshold was very conservative and set at 1.35%.
- Patient characteristics were similar in both groups.
-
Performed both intention to treat (ITT) and per-protocol analyses.
- ITT analysis represents patients randomized to a particular study group, whether they adherent to the study protocol or not.
- Per-protocol analysis represents patients randomized to a particular study group and who were adherent to the study protocol.
-
Performed sensitivity analysis for missing outcomes.
- Systematic evaluation of uncertainty in data that incorporates varying probability estimates for the primary end-point.
- Planned post hoc safety analysis of subpopulation of patients with zero YEARS criteria (those who had a change in their diagnostic strategy).
- The prevalence of PE was similar to other studies that investigated patients with low and moderate clinical probability.
Limitations:
- Though it was an international study, the population likely represents a majority French cohort as 18 participating EDs were from France and only two EDs were from Spain.
- Though YEARS criteria are simple, the additional combination of PERC algorithm and age-adjusted D-dimer increases complexity.
- Nonconsecutive enrollment by ED physicians contributes to selection bias.
- 37 patients (2.6%) had missing primary end-point
-
Did not perform a complete case analysis
- The analysis evaluates only patients with complete data.
- There is no mention of how they handled patients with intermediate probability diagnosis of V/Q scan.
Discussion:
-
Combining multiple decision instruments:
- The YEARS score has only 3 criteria, and cutoff values are simple to remember.
- The PERC algorithm has 8 criteria, and age-adjustment for D-dimer requires yet another step.
- The combination of all three may be too cumbersome for some clinicians.
- Although anecdotally, many clinicians are already combining these decision instruments.
- The trial provides evidence that this approach is safe.
-
Post hoc safety analysis on the subgroup of patients with YEARS score of zero:
- There were no missed PEs within the intervention group.
- Absolute reduction in chest imaging was 14% in the intervention group.
- 1 in 7 patients avoided chest imaging in the intervention group.
-
Noninferiority Trials: (Covered on REBEL EM)
- Establishing noninferiority is practical when the intervention provides an advantage over the standard treatment (i.e., limiting exposure to radiation and reducing the utilization of resources).
- The noninferiority threshold was very cautious at 1.35% in this trial.
- It is easier to show noninferiority if the threshold is large.
-
Investigators included an ITT analysis and per-protocol analysis.
- ITT is more reflective of real-world experience, but protocol violations can narrow the difference between groups and lead to a false conclusion of noninferiority. (Mauri 2017)
- Per-protocol analysis may be preferable in a noninferiority trial but can also introduce post-randomization bias. (Mauri 2017)
- Including both analyses increases the validity of the results.
-
Nonconsecutive enrollment performed by ED physicians.
- While this approach is pragmatic, it can lead to selection bias.
- It’s unclear how many patients were missed or whether patients enrolled represent a typical cohort compared to those missed.
-
Dealing with loss to follow up:
- Investigators assumed 37 patients with omitted info missed the primary end-point for the ITT analysis due to a low prevalence rate (<2%).
- However, patients lost to follow-up have worse outcomes. (Guyatt 2015)
- 37 patients represent only 2.6% of the trial but more than four times the 9 patients who met the primary end-point for the entire investigation.
- The fragility index of this paper was zero.
- One additional patient who met the primary end-point in the intervention arm would push this group beyond the narrowly set noninferiority margin.
- Researchers revealed little information about these patients. Were they balanced across both groups? Did they have the same risk of VTE?
- The results were validated by the sensitivity analysis. However, the addition of a complete case analysis might have further bolstered results.
-
How were patients with intermediate probability V/Q scans managed?
- The PIOPED trial demonstrated 33% of patients with intermediate probability V/Q scan had confirmed PE on CTPA.
- However, only a minority of patients with confirmed PE had a high probability V/Q scan.
- We might assume patients with intermediate probability V/Q scan had CTPA, but the authors do not explicitly state this.
-
FEU vs. DDU:
- FEU is the common D-dimer assay with a 500 ng/mL cutoff.
- DDU has a cutoff of 250 ng/ml.
- For patients with a YEARS score = 0, the FEU cutoff is 1000 ng/mL, and DDU cutoff is 500 ng/mL.
- For patients with a YEARS score ≥ 1, the FEU cutoff is 500 ng/mL, and DDU cutoff is 250 ng/mL.
- Age–adjustment for FEU is 10 X age, and DDU is 5 X age.
Author’s Conclusions: “Among ED patients with suspected PE, the use of the YEARS rule combined with the age-adjusted D-dimer threshold in PERC-positive patients, compared with a conventional diagnostic strategy, did not result in an inferior rate of thromboembolic events.”
Our Conclusions: We agree with the author’s conclusion. The YEARS criteria in combination with age-adjusted D-dimer is noninferior to age-adjusted D-dimer alone and has the potential to decrease chest imaging in PERC-positive patients with low or moderate clinical probability for PE.
Clinical Bottom Line:
- In patients with low clinical pretest probability and zero YEARS criteria, use a 1000 ng/mL FEU D-dimer threshold.
- In patients with low to moderate clinical pretest probability, adjust the D-dimer for age.
For More Thoughts on This Topic Checkout:
PERC:
- REBEL EM: Is It PROPER to PERC It Up
- EM Literature of Note: Using PERC & Sending Home Pulmonary Emboli For Fun and Profit
- EMNerd (EMCrit): The Case of the Diagnostic Absurdity
- PulmCCM: Ruling out PE in the ED – Critical Analysis of the PROPER Trial
- PulmCCM: PERC Can Safely Rule Out Pulmonary Embolism in ED Setting
- Core EM: PE Rule-Out Criteria RCT
YEARS:
- REBEL EM: The YEARS Study – Simplified Diagnostic Approach to PE
- EM Lit of Note: YEARS, But Wells
Age-Adjusted D-dimer:
- Core EM: Age Adjusted D-dimer in PE – The ADJUST-PE Trial
- REBEL EM: PEGeD Study – Is It Safe to Adjust the D-Dimer Threshold for Clinical Probability?
- REBEL EM: Age Adjusted D-dimer Testing
- The Bottom Line: PEG-eD
- St. Emlyn’s: Level Pegging? JC and the PEGeD Study
- JournalFeed: Is it Time to Adjust D-Dimer Thresholds to Our Clinical Pretest Probability?
- The SGEM: SGEM #282 – It’s All ‘Bout That Bayes, ‘Bout That Bayes – No Trouble – In Diagnosing Pulmonary Embolism
ACEP Clinical Policy:
- REBEL EM: ACEP Clinical Policy on Acute VTE 201
- FOAMCast: Pulmonary Embolism Risk Stratification and ACEP Clinical Policy
References:
- Kline JA, Mitchell AM, Kabrhel C, Richman PB, Courtney DM. Clinical criteria to prevent unnecessary diagnostic testing in emergency department patients with suspected pulmonary embolism. J Thromb Haemost. 2004;2(8):1247-1255. [Link is here]
- Righini M, Van Es J, Den Exter PL, et al. Age-adjusted D-dimer cutoff levels to rule out pulmonary embolism: the ADJUST-PE study [published correction appears in JAMA. 2014 Apr 23-30;311(16):1694]. JAMA. 2014;311(11):1117-1124. [Link is here]
- van der Hulle T, Cheung WY, Kooij S, et al. Simplified diagnostic management of suspected pulmonary embolism (the YEARS study): a prospective, multicentre, cohort study [published correction appears in Lancet. 2017 Jul 15;390(10091):230]. Lancet. 2017;390(10091):289-297. [Link is here]
- American College of Emergency Physicians Clinical Policies Subcommittee (Writing Committee) on Thromboembolic Disease:, Wolf SJ, Hahn SA, et al. Clinical Policy: Critical Issues in the Evaluation and Management of Adult Patients Presenting to the Emergency Department With Suspected Acute Venous Thromboembolic Disease. Ann Emerg Med. 2018;71(5):e59-e109. [Link is here]
- Mauri L, D’Agostino RB Sr. Challenges in the Design and Interpretation of Noninferiority Trials. N Engl J Med. 2017;377(14):1357-1367. [Link is here]
- Guyatt G, Rennie D, Meade M, Cook D. Users’ Guides To The Medical Literature. 3rd ed. McGraw-Hill Education; 2015. [Link is here]
- PIOPED Investigators. Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). JAMA. 1990;263(20):2753-2759. [Link is here]
Post-Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The Pragmatic Combination of YEARS Score and Age-Adjusted D-Dimer appeared first on REBEL EM - Emergency Medicine Blog.
