
 Background: Oral factor Xa inhibitors (FXi) have been associated with major and fatal bleeding events, including intracranial hemorrhage (ICH). In randomized controlled trials, both rivaroxaban and apixaban are associated with ICH at rates that range from 0.1 to 4% (Agnelli 2013, Granger 2011, Investigators 2010, Patel 2011). While these studies did not specifically set out to identify ICH in their primary outcomes, each one found an association with an FXi and ICH.
Background: Oral factor Xa inhibitors (FXi) have been associated with major and fatal bleeding events, including intracranial hemorrhage (ICH). In randomized controlled trials, both rivaroxaban and apixaban are associated with ICH at rates that range from 0.1 to 4% (Agnelli 2013, Granger 2011, Investigators 2010, Patel 2011). While these studies did not specifically set out to identify ICH in their primary outcomes, each one found an association with an FXi and ICH.
FXi reversal is considered to be a central part of management in patients presenting with ICH. This is typically accomplished with four-factor prothrombin complex concentrate (4F-PCC). In recent years, andexanet alfa (AA) has emerged as an alternate option. AA is the first and only FDA-approved selective reversal agent for the treatment of life-threatening bleeding associated with oral FXi therapy. However, its approval is based largely on phase II studies sponsored by the pharmaceutical company, Portola Pharmaceuticals, which manufactures the reversal agent (Siegal 2015). The existing studies also contain significant biases; they are small, unblinded, and their primary outcomes are not patient-centered (ie. Siegal investigated factor Xa activity).
Though a consensus statement by the American College of Cardiology recommended AA over 4F-PCC in the treatment of FXi associated ICH, there have been no prospective studies to suppose the use of one agent over the other.
Clinical Question: How does andexanet alfa compare to four-factor PCC in the treatment of patients with FXi associated intracranial hemorrhage?
Article: Ammar AA, Ammar MA, Owusu KA, et al. Andexanet Alfa Versus 4-Factor Prothrombin Complex Concentrate for Reversal of Factor Xa Inhibitors in Intracranial Hemorrhage. Neurocrit Care. 2021;35(1):255-261. doi:10.1007/s12028-020-01161-5 (PMID: 33403588)
What They Did:
- This is a retrospective observational study done at a single center
- Included a consecutive series of adult patients admitted to Yale New Haven Health System from July 2018 to April 2019 presenting with life-threatening ICH in the setting of oral FXi therapy.
- Patients were treated with one dose of either AA or 4F-PCC
- Patients had a CT scan at 6 and 24 hours post-administration of AA or 4F-PCC
-
Data extracted from electronic medical records including:
- Patient demographics
- Clinical information
- Imaging information
- Laboratory information
- Each brain imaging study was independently reviewed by three experienced providers blinded to the treatments and outcome and rated for stability
-
Stability was defined as similar amount of blood from one scan to the next
-
For intraparenchymal hemorrhages, the volume was calculated via ABC/2 volume estimation method
- Similar amount of blood was defined as a volume growth of <6 mL OR 33% from baseline CT
-
For intraparenchymal hemorrhages, the volume was calculated via ABC/2 volume estimation method
-
Thrombotic events were identified via chart review, and included:
- Upper and lower extremity DVT
- Pulmonary embolism
- Ischemic stroke
- Myocardial infarction
- Catheter-associated thrombosis
- Other thromboses documented between reversal agent and hospital discharge
Population:
- Inclusions: Adult patients with a life-threatening traumatic or spontaneous intraparenchymal, subarachnoid, subdural hemorrhages and other intracranial bleeds in the setting of FXi (apixaban or rivaroxaban) use
- Exclusions: Patients who received both AA and 4F-PCC during the same hospitalization
Intervention:
- AA versus 4F-PCC for oral FXi use in acute ICH
Outcomes:
-
Primary outcome: Stable head CT at 6 hours and 24 hours post-administration of reversal agent
-
Defined as
- No significant increase in volume of bleed (<6mL or 33% from baseline volume) for IPH
- Stable CT as determined by “an experienced provider” for all other bleeds
-
Defined as
-
Secondary outcomes:
- Good functional outcome at discharge (Modified Rankin Score of 0-3)
- In-hospital thrombotic events after reversal therapy
- Short-term mortality (in-hospital mortality or discharge to hospice)
- Length of stay (hospital and ICU)
- Disposition on discharge
Results:
-
Total of 44 patients enrolled
-
16 cases were traumatic
- 75% multicompartmental bleeds
-
28 cases were spontaneous hemorrhages
- 68% intraparenchymal bleeds
- 28 cases received AA
- 16 cases received 4F-PCC
-
16 cases were traumatic
- No difference between groups regarding type of bleed
- More patients received aspirin therapy in AA group 39%, vs 6% in the 4F-PCC group.
- Average GCS on admission between both groups was 14
- Average kidney function was within normal limits between both groups
Critical Results:
-
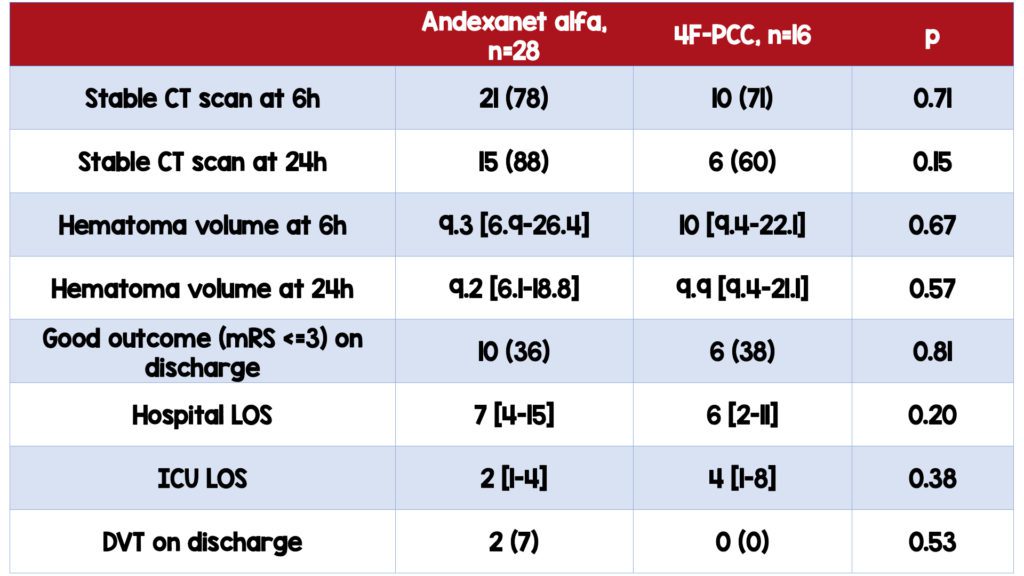
No significant difference in the proportion of patients with stable neuroimaging assessment between the two groups
- Remained the same when adjusted for age and sex
- No statistically significant difference in 24-hour CT stability between the two groups (88% AA v 60% 4FPCC)
- No significant difference in the degree of achieved hemostasis based on hematoma volume between the two groups
-
No significant difference in patients with good outcomes upon discharge between the two groups
- 36 % (AA) v. 38% (4F-PCC)
-
No significant difference in the incidence of thrombotic events
- 2 in AA group, 0 in 4F-PCC group
- ICU length of stay was higher in 4FPCC group (2 days v. 4 days, p=0.38), although not statistically significant
- Incidence of VTE was higher in the AA group (7 v. 0, p=0.58), although not statistically significant
Strengths:
- Neuroimaging reviewed by 3 blinded physicians
- Thromboembolic events supported by imaging/laboratory studies
- Data was adjusted for age and sex
- Consecutive enrollment
Limitations:
- Single-center, retrospective chart review
- Small sample size (n=44)
-
Largely homogenous group; limits generalizability
- 86% White
- Average age of 78
- >60% male (61% and 69% for AA v. 4FPCC, respectively)
- Patients in AA group had more comorbid conditions, although this is not statistically significant
- Primary outcome is a surrogate, disease-centered outcome as opposed to a patient-centered outcome
- No formal definition for worsening bleed outside of intraparenchymal hemorrhages
- More patients in the AA group also received concomitant aspirin therapy (39% vs. 6%) which may bias the results
- No discussion of chart review methodology
- Unclear if there were multiple data extractors or single
- No discussion of training of the data extractors
- No treatment blinding
- No randomization due to retrospective study design
Discussion:
-
This is a retrospective observational study
-
Often need larger sample sizes
- N in this study is 44
- Cannot determine causation, only association
-
Often need larger sample sizes
-
Selection bias
- No way to know why one patient was selected for one treatment over another
- In theory, only those who had no chance for survival could have been given 4FPCC, while those who were thought to be most salvageable were given the AA. This would actually mean that despite biasing of the study, AA could be worse and vice versa.
-
No randomization in medications chose
- No discussion as to what was standard practice, however, data is more heavily weighted toward AA (28 v 16 in comparison to 4F-PCC)
- The sample size is small, making it hard to find any statistically significant differences
-
Baseline characteristics were unbalanced
- More patients in the AA group also received concomitant antiplatelet therapy (39%)
- AA group had more comorbidities
- AA group had more spontaneous ICH and larger proportion of IPH in comparison to 4F-PCC group
-
CT stability at 24h were different between the two groups (88% AA v 60% 4FPCC), although not statistically significant (p=0.15)
- A larger, properly powered study may find this difference to be real, statistically and clinically significant.
-
Incidence of VTE was higher in the AA group (7 v. 0, p=0.58), although not statistically significant
- Larger studies should be done to assess for a possible statistically significant difference that ultimately affects patient safety
-
Previous studies have evaluated AA and 4FPCC separately, but not in comparison
-
The only study comparing the two was with regards to thrombotic events (Kimpton 2019)
- AA was found to have higher VTE events than 4FPCC
-
This was, however, not done specifically in terms of ICH
- Studies regarding ICH should be done to assess whether this difference exists across this specific patient population
-
The only study comparing the two was with regards to thrombotic events (Kimpton 2019)
- Average GCS is 14 in both groups; so, results cannot be generalized to more severe bleeds
- The overall kidney function in this group is also normal; therefore, this study cannot be generalized to those with poor kidney function
Author Conclusion: “We found no significant difference in the degree of achieved hemostasis based on CT stability, functional outcomes at discharge and thrombotic events during admission when comparing AA and 4F-PCC for the reversal of oral FXi in the setting of ICH. Large observational and randomized studies comparing the efficacy and safety of AA and 4FPCC in patients with acute intracranial hemorrhage are needed.”
Our Conclusion: This study did not demonstrate any difference in the surrogate endpoint of ICH growth at 6 and 24 hours based on reversal agent. However, the study’s flaws render the data difficult to interpret or apply clinically. A larger, prospective multi-centered study with diverse population is needed to truly compare the efficacy and safety between AA and 4FPCC for FXi reversal in the setting of ICH
Potential to Impact Current Practice: 4F-PCC is cheaper and more widely available than AA ($5670/patient, compared to $22,120-$49,500/patient); if there is truly no difference in outcome, we can use the more cost-effective therapy for patients. Again, better studies are needed to definitively make a recommendation.
Clinical Bottom Line:
This limited study found no statistically significant difference in stability of oral FXi related ICH after the administration of AA or 4F-PCC. However, the inherent potential bias and small participant numbers limit generalizability and therefore larger prospective studies are needed.
References:
- Agnelli G, Buller HR, Cohen A, et al. Oral apixaban for the treatment of acute venous thromboembolism. N Engl J Med. 2013;369:799–808. (PMID 23808982)
- Granger CB, Alexander JH, McMurray JJ, et al. Apixaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2011;365:981–92. (PMID 21870978)
- Investigators E, Bauersachs R, Berkowitz SD, et al. Oral rivaroxaban for symptomatic venous thromboembolism. N Engl J Med. 2010;363:2499–510. (PMID 21128814)
- Patel MR, Mahaffey KW, Garg J, et al. Rivaroxaban versus warfarin in non- valvular atrial fibrillation. N Engl J Med. 2011;365:883–91. (PMD 21830957)
- Siegal DM, Curnutte JT, Connolly SJ, et al. Andexanet alfa for the reversal of factor Xa inhibitor activity. N Engl J Med. 2015;373:2413–24. (PMID: 26686866)
- Kimpton M, Siegal DM. Evidence-Based Minireview: Mortality and thrombosis in patients receiving prothrombin complex concentrates or andexanet alfa for the management of direct oral factor Xa inhibitor-associated major bleeding. Hematol Am Soc Hematol Educ Progr. 2019;2019:204–8. (PMID: 31808849)
Guest Post By:

Sarah Aly, DO
PGY-2, Emergency Medicine Resident
Saint Joseph’s University Medical Center, Paterson New Jersey
Twitter: @alyalyoxenfree_

Marco Propersi, DO FAAEM
Assistant Professor, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson New Jersey
Twitter: @marco_propersi
Steven Hochman, MD FACEP
Associate Professor, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson New Jersey
Twitter: @hochmast
Post-Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Andexanet Alfa Vs. Four-Factor PCC: Is Andexanet Alfa Worth The Hype? appeared first on REBEL EM - Emergency Medicine Blog.

