 Background: Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are commonly seen in the ED. AECOPD is characterized by a change in the patient’s baseline dyspnea, cough or sputum purulence. While there are a number of causes for exacerbations, infectious etiologies are one of the most common. Viral and bacterial pathogens are frequently responsible though it is unclear how much of a role each plays. Treatment of outpatient AECOPD with antibiotics is common based on extrapolation of data from the inpatient setting though there is inconsistent evidence for benefit in mild to moderate exacerbations (Vollenweider 2012, van Velzen 2017). If antibiotics are prescribed, the optimal duration is also unclear though shorter courses (< 6 days) have been shown to be as effective as longer courses (> 7 days) with a lower side effect profile (Stolbrink 2018).
Background: Acute exacerbations of chronic obstructive pulmonary disease (AECOPD) are commonly seen in the ED. AECOPD is characterized by a change in the patient’s baseline dyspnea, cough or sputum purulence. While there are a number of causes for exacerbations, infectious etiologies are one of the most common. Viral and bacterial pathogens are frequently responsible though it is unclear how much of a role each plays. Treatment of outpatient AECOPD with antibiotics is common based on extrapolation of data from the inpatient setting though there is inconsistent evidence for benefit in mild to moderate exacerbations (Vollenweider 2012, van Velzen 2017). If antibiotics are prescribed, the optimal duration is also unclear though shorter courses (< 6 days) have been shown to be as effective as longer courses (> 7 days) with a lower side effect profile (Stolbrink 2018).
Article: Messous S et al. Two-day versus seven-day course of levofloxacin in acute COPD exacerbation: a randomized controlled trial. Ther Adv Resp Dis 2022; 16: 1-10: PMID: 35657073
Clinical Question: Is a 2-day course of levofloxacin non-inferior to a 7-day course for clinical cure in patients with a COPD exacerbation?
Population: Adult patients > 45 years of age with a smoking history of at least 10 pack years with a clinical diagnosis of mild-to-severe COPD with an acute exacerbation defined as worsening respiratory symptoms resulting in the need for additional therapy.
Outcomes:
- Primary: Clinical cure: Complete resolution of acute signs and symptoms associated with exacerbation and no recurrences at 30 days follow up.
-
Secondary:
- Need for additional antibiotics
- ICU admission rate
- Exacerbation free interval (EFI)
Intervention: Levofloxacin 500 mg PO Q24 X 2 days
Control: Levofloxacin 500 mg PO Q24 X 7 days
Design: Randomized, double-blind, placebo controlled, multicenter non-inferiority trial with the non-inferiority margin set at 10%.
Excluded: Clinical evidence of hemodynamic compromise with need for vasoactive medications, immediate need for endotracheal intubation, pneumonia, previous adverse reaction to the study drug, antibiotic treatment in the previous days, pregnancy or lactation, severe renal or hepatic impairment, lung disease other than COPD, alcohol or drug use.
Primary Results
-
- 712 patients were screened, and 310 patients were randomized.
- Sputum sampling adequate in 58.3% of patients
- Pathogens isolated
- 2-day course: 44
- 7-day course: 37
- Isolated pathogens consistent between groups
- Pathogens isolated
- All patients were treated with steroids
Critical Findings:
- The 2-day course of levofloxacin met the pre-specified non-inferiority margin for the primary outcome of clinical cure rate
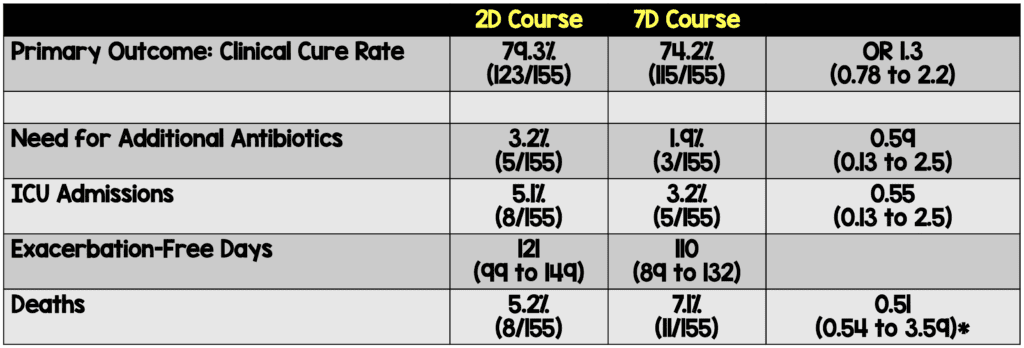
- *Calculation appears to be in error in both body and table
- There was no significant difference in adverse events between the two groups.
Strengths:
- Asks a clinically important question with limited prior high-quality data
- Baseline characteristics are well-balanced between the two groups
- Randomization and blinding were adequately performed
- Reported 100% follow up rate for the primary and secondary outcomes
- To ensure blinding active drug and placebo tablets were encapsulated for identical appearance
Limitations:
- Patients were not enrolled consecutively introducing a selection bias.
- The primary outcome is subjective. There is no mention of the training given to outcome assessors or information regarding inter-rater reliability.
- Over 200 patients were excluded from the study due to patient choice or for an unreported reason. This biases the results.
- The groups were slightly imbalanced in terms of hospitalizations beyond the 48 hours observation period with more patients in the 7-day arm being hospitalized for a longer interval.
Discussion
- The primary outcome is subjective in nature. This is important to note in a non-inferiority study as we have previously discussed (REBEL EM)
- All patients were observed in the ED for 48 hours. This is not standard practice in most places.
- 100% follow up at one year seems almost impossible.
- The major question that remains unanswered is whether patients with mild-to-moderate AECOPD (those appropriate for discharge home) should be treated with antibiotics at all
- It’s unclear how many AECOPD are caused by bacteria. Even when bacteria are cultured from sputum, they may not be “responsible” as COPD patients’ lungs can be colonized with bacteria.
- Corticosteroids have consistently shown to be of benefit in this group.
- Data on antibiotics is inconsistent in terms of immediate improvement as well as recurrent exacerbations.
- Studies do show a signal for harm with antibiotic therapy due to side effects.
- Despite the absence of clear evidence of benefit for antibiotics, if antibiotics are prescribed, they should be provided for the shortest duration possible.
Authors Conclusions: “Levofloxacin once daily for 2 days is not inferior to 7 days with respect to cure rate, need for additional antibiotics and hospital readmission in AECOPD. Our findings would improve patient compliance and reduce the incidence of bacterial resistance and adverse effects.”
Our Conclusions: We agree that this study demonstrates non-inferiority of a 2-day course of levofloxacin to a 7-day course. However, the trial has a number of issues including the subjectivity of the outcome measure which may bias the results. Subsequent studies should focus on generating high-quality data looking at short-course antibiotics versus no antibiotics.
Bottom Line: It remains unclear if mild to moderate AECOPD benefit from antibiotics but, if you are going to prescribe them, a short course appears to be adequate.
For More on This Topic Checkout:
References:
- Vollenweider DJ et al. Antibiotics for exacerbations of chronic obstructive pulmonary disease. Cochrane Database Syst Rev 2012. PMID: 23235687
- van Velzen P et al. Doxycycline for outpatient-treated acute exacerbations of COPD: a randomised double-bind placebo-controlled trial. Lancet Respir Med 2017; 5: 492-9. PMID: 28483402
- Stolbrink M et al. Does antibiotic treatment duration affect the outcomes of exacerbations of asthma and COPD? A systematic review. Chron Respir Dis 2018; 15(3): 225-40. PMID: 29232988
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Antibiotics in COPD Exacerbations – 2 days vs 7 days appeared first on REBEL EM - Emergency Medicine Blog.
