 You are working at a Level 1 Trauma Center; a 35-year-old female arrives via EMS from the scene of a motor vehicle accident. She was an unrestrained passenger, ejected 50 feet. She was hypotensive and hypoxic on scene with concern for head injury with a GCS of 7.
You are working at a Level 1 Trauma Center; a 35-year-old female arrives via EMS from the scene of a motor vehicle accident. She was an unrestrained passenger, ejected 50 feet. She was hypotensive and hypoxic on scene with concern for head injury with a GCS of 7.
She is clearly in shock on arrival with weak pulses, clammy skin, and a BP of 80/50mmHg, HR 140, sats 85%. She is intubated, a chest tube is placed on the left (with improvement in O2 sats to 95%), and a pelvic binder is placed for suspected pelvic fracture. eFast demonstrates free fluid in the pelvis. Massive Transfusion Protocol (MTP) has been activated appropriately, and despite rapid delivery of 4 units Packed Red Blood Cells (PRBCs), 2 units of Fresh Frozen Plasma (FFP) and 1 pack of Platelets, she remains hypotensive, with presumed hemorrhagic shock.
The patient is destined for the OR, but you ask yourself, in traumatic hemorrhagic shock, is there a role for vasoactive agents?
Vasoactive Agents: A Historical Perspective on Their Use in Hemorrhagic Shock
Vasoactive medications are one of the pillars of management of shock in Emergency Departments[1]. Inopressors, namely Norepinephrine and Epinephrine, are the two most commonly used pressors in US Emergency Departments, used most frequently to treat distributive shock[1]–[3]. More recently, ‘push dose pressors’ have become increasingly recognized as a means to achieve rapid hemodynamic improvement in unstable patients, and pre-emptively during intubation in patients at risk of peri-intubation arrest[4].
Vasopressin History and Action:
- Arginine Vasopressin (AVP) is an endogenously released hormone, also known as anti-diuretic hormone (ADH). The direct vasoconstricting effect of Vasopressin occurs due to its agonist activity at the V1 receptor, causing direct activation of Phospholipase C, activation of the phosphoinositide pathway and vasoconstriction of smooth muscle[5].
- Vasopressin also acts as an ‘indirect’ vasoconstrictor, enhancing the sensitivity of the vasculature to circulating catecholamines[6].
- Despite the proven comparable efficacy of Vasopressin to its catecholamine derived counterparts in the management of ‘hypotensive shock’, Vasopressin is largely considered a second line agent[1], [7].
Why Don’t we use Pressors in Hemorrhagic Shock?
Unlike distributive shock, the current cornerstones of the management of hemorrhagic shock are timely hemorrhage control with simultaneous balanced volume resuscitation[8], [9]. Traditionally, vasoactive agents have been contraindicated in early hemorrhagic shock, secondary to their deleterious consequences and increased mortality risk[10]. ATLS does not recommend the use of vasopressors.
Sperry et al, (2008, Journal of Trauma), published a secondary analysis of a prospective multicenter cohort study[10]. Mortality rates were compared between patients who did and did not receive early vasopressor therapy (within 12 hours of injury). Among 921 patients, overall study mortality was 12%, and the use of early vasopressors was associated with an increased mortality risk at 12h (hazard ratio 1.81) and 24 hours (hazard ratio 2.15). This study is limited by the fact that it was a secondary analysis of a prospective cohort and was not originally designed to answer the specific hypothesis tested.
Vasopressin was the only vasopressor in that study not associated with increased mortality on logistic regression.
Given the retrospective nature of these studies, the use of vasopressors may represent a marker of increased severity of illness rather than a direct contributor to adverse outcomes
Why Vasopressin Might Make Sense in Trauma Patients with Hemorrhagic Shock
- Arginine Vasopressin is a hormone secreted by the posterior pituitary. It is released in response to increased serum osmolality or hypotension. It is essential during hemorrhagic shock, and up to 20% of posterior pituitary stores can be released imminently during the initial stage of blood loss and during the initial phase of hemorrhagic shock[11][12].
- As shock progresses, Vasopressin, which has a relatively short half-life (10 – 35 minutes), may become depleted[13]. Low levels of circulating Vasopressin are associated with catecholamine resistance, vasoplegia and increased venous capacitance[14].
- It is this theory of impaired baroreceptor mediated secretion and overall depletion with shock progression that underpins Vasopressin’s use as an adjunct to volume repletion in trauma patients with hemorrhagic shock.
- The addition of exogenous low dose Vasopressin is thought to dramatically improve vascular tone in shock states associated with Vasopressin deficiency[15].
- There is also evidence that early use of Vasopressin in the trauma patient with hemorrhagic shock and traumatic brain injury (TBI) may assist in rapidly correcting Cerebral Perfusion Pressure (CPP) and Improve cerebrovascular compliance[16].
Evidence for the use of Vasopressin in Hemorrhagic Shock Patients
The AVERT-Shock trial: This was a single center Randomized Clinical Trial examining the effects of Low Dose Vasopressin (LDV) on the early resuscitation of patients with trauma and hemorrhagic shock[17].
A. Sims et al., “Effect of Low-Dose Supplementation of Arginine Vasopressin on Need for Blood Product Transfusions in Patients With Trauma and Hemorrhagic Shock: A Randomized Clinical Trial,” JAMA Surg, Aug. 2019.
- Randomized, double-blind placebo-controlled trial
- Single Level 1 Trauma Center
- Enrollment period: May 1st 2013 – May 31st 2017
- Inclusion criteria: Adult trauma patients (18-65) who received at least 6 units of any blood product within 12 hours of injury
- Exclusion criteria: Prehospital cardiopulmonary resuscitation, emergency department thoracotomy, corticosteroid use, chronic renal insufficiency, coronary artery disease, traumatic brain injury requiring any neurosurgical intervention, pregnancy, prisoner status, or Vasopressin administration before enrollment
- After administration of a Vasopressin bolus (4U) or placebo, participants received Vasopressin (0 – 0.04 U/min) or placebo for 48 hours to maintain a mean arterial blood pressure of at least 65 mm Hg
- Vasopressin only given after the attainment of definitive hemorrhage control
- The study infusion was titratable to the patient’s hemodynamic response
- If vasopressors were needed, neo-synephrine, norepinephrine, and/or epinephrine were used. All vasopressor treatments were titrated and stopped before tapering the study infusion.
- All health care professionals were blinded to treatment assignment
Primary Outcome:
- Total volume of blood product transfused within 48 hours and included Packed Red Blood Cells (PRBCs), Fresh Frozen Plasma (FFP) and Platelets but NOT Cryoprecipitate
Secondary Outcomes:
- Total volume of crystalloid transfused
- Vasopressor requirements
- Secondary complications
- 30-day mortality
Results:
- A total of 100 patients were enrolled, 41 patients randomized to Vasopressin, 51 to placebo
- 93 men and 7 women; median age 27 years [IQR, 22-25 years]
- 79 cases were penetrating trauma, 21 were blunt
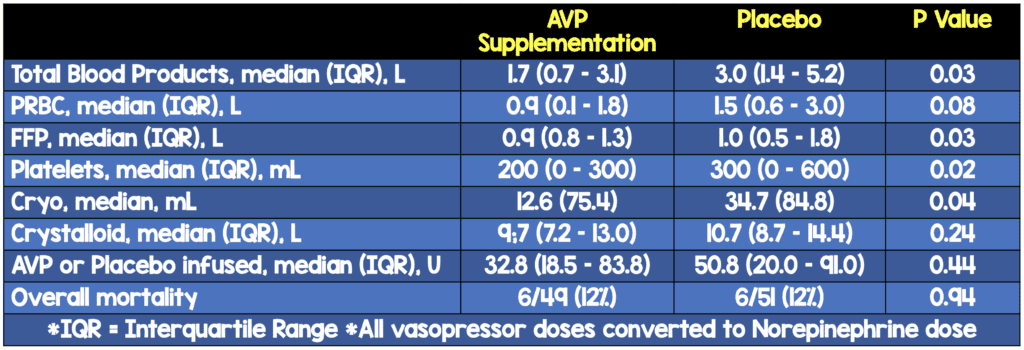
- Low Dose Vasopressin (LDV) was associated with significantly less volume of FFP (median, 0.9 vs 1.0 L), platelets (median, 200 vs 300 mL), and cryoprecipitate (mean 12.6 vs 34.7 mL) and significantly less cumulative volume of all blood products with an estimated median difference of −1.00 L (95% CI, −2.03 to 0.00 L; P = .03)
- Although LDV did not affect the overall complication rate, it was associated with a decreased rate of DVTs (10 of 49 [20%] vs 20 of 51 [39%]
- Vasopressin did not significantly influence resuscitation-related complications such as acute respiratory distress syndrome, length of mechanical ventilation, acute kidney injury, or time needed for damage control of open abdomen.
- Vasopressin also did not significantly affect median LOS in the intensive care unit or hospital days, risk of operative death or overall mortality
Critical Results:
Strengths:
- Answers a clinically important question
- Answers specific question of vasopressin use based on physiologic theory of vasopressin depletion
- Groups were well balanced before randomization in terms of baseline characteristics and severity of illness
- Clinical team, research personnel, patients’ families and patients were blinded to group assignment for the duration of the trial
- Intention to Treat Analysis: Reliable estimate of true treatment effectiveness by replicating ‘real world’ conditions
Weaknesses:
- Single center
- Penetrating trauma predominance may affect generalizability of results
- Cohort of 100 underpowered to detect differences in many clinically relevant outcomes
- Low recruitment numbers may be consistent with community distrust of clinical trial enrollment in an inner-city population
- Both groups received relatively large amounts of crystalloid fluids within 48hrs (median 9.7 and 10.7L), which could have affected results of this study
- Cryoprecipitate was not included in the cumulative product volume but was analyzed separately
- The total dose of AVP infused varied depending on the patient’s hemodynamic stability
- Underpowered to detect significant differences in many clinically relevant outcomes. Similarly, given the small sample size, the authors did not adjust for multiple comparisons. As such, a larger study will be needed to determine the effect of AVP on acute kidney injury, acute respiratory distress syndrome, mechanical ventilation, and LOS.
Authors Conclusions:
“Low-dose AVP during the resuscitation of trauma patients in hemorrhagic shock decreases blood product requirements. Additional research is necessary to determine whether including AVP improves morbidity or mortality.”
Why are the Findings of the AVERT-Shock Trial Clinically Important?
- Hemorrhage is the leading cause of death in trauma patients[17]. Resuscitation with blood products is the criterion management standard. However, blood products are a finite, precious resource.
- Concerns have increased about the possible negative immunomodulatory effects of blood product transfusion[18]. These immunomodulatory effects may go some way to explain the peculiar finding of fewer DVTs with LDV; several studies have shown increased thromboembolism risk with blood transfusion in a dose dependent fashion[19]. However, due to low numbers in this study, this finding could represent statistical noise. Further studies are needed before making any definitive conclusions.
- AVERT clearly demonstrates that LDV administration in patients with hemorrhagic shock significantly decreased the use of all blood products and improved fluid balance at 48 hours.
- AVERT demonstrates that the addition of a vasopressor agent in the resuscitation (albeit after definitive hemorrhage control was achieved) did not result in higher complication rates or mortality; it thus challenges the traditional dogma that there is no place for Vasopressors in bleeding with hypotension.
- Like any good clinical trial, it also generates as many if not more questions than it answers. What is the optimal timing of Vasopressin initiation? Is there a role of push dose Vasopressin in this patient cohort? Are the above findings applicable to a cohort of patients presenting with primarily blunt trauma? AVERT will likely serve merely as the beginning for larger studies addressing these issues.
Clinical Bottom Line:
- The use of Vasopressin as an effective adjunct to the resuscitation of hemorrhagic shock is physiologically plausible
- The optimal dose and timing of LDV administration is still not clear based off this one single center study.
- The AVERT-Shock trial demonstrates that the use of Low Dose Vasopressin (LDV) in hemorrhagic shock was not associated with complications or increased mortality. However, AVERT-Shock was underpowered to detect a number of clinically significant secondary outcomes. Larger studies are needed to determine the effect of LDV on these outcomes.
- In a level one trauma center with access to a massive transfusion protocol, the addition of low dose vasopressin to a patient with continued hypotension despite aggressive administration of blood products is reasonable.
- The AVERT-Shock trial does not inform our practice in resource limited environments that do not have access to massive transfusion. There is no data currently available to show any benefit / harm of using Vasopressin earlier in the resuscitation of trauma patients prior to resuscitation with blood products.
Guest Post By:

Expert Peer Review

David Morris, MD
Trauma Surgeon
Intermountain Medical Center
Salt Lake City, UT
Twitter: @traumadmo
Pressors in trauma? Heresy! At least that is what I was taught throughout surgical residency and into my fellowship. Like many of the principles we think we know about medical care, it turns out that the inherited dogma we carry around in our heads is often based more on the opinions and practice patterns of those that trained us than they are on scientific evidence. While much of what we learn is appropriate and helpful for patient care, some of it is not. Unquestioning acceptance creates clinical or research blind spots, but unbridled iconoclasm discounts the years of hard-earned experience of our mentors and teachers. How to find the balance?
As is often the case, the truth about pressors in resuscitation of hemorrhagic shock, probably lies somewhere in the nuanced area between absolute prohibition and indiscriminate liberality. The study reviewed here demonstrates the complexity of modern medicine – the more we learn, the more we see how much more have to learn. Dr. Sims and colleagues demonstrated in this elegant trial that there may be a role for replacement of homeostatic levels of vasopressin during resuscitation of the massively bleeding patient. They were able to demonstrate that the use of vasopressin in this way was independently associated with reduced transfusion requirements without increasing the dreaded adverse outcomes of pressor therapy that were often quoted (anecdotally, of course) to me during my training.
So what do we do with this information? Is reduced blood transfusion in and of itself a reasonable enough endpoint to justify practice change? It stands to reason that reduced transfusion requirement is a good surrogate outcome for the only outcome that providers and patients care about, namely improved survival. But this study wasn’t powered to show a survival benefit, nor was it intended to show a difference in complications of pressor therapy. If I’m the one bleeding in the ER, would it matter to me that I get fewer units of blood product transfused if I ultimately survive my injuries?
Avoiding blood product exposure is, undoubtedly, a worthy goal, one which we sometimes forget in the era of “safe” transfusions. The providers that practiced in the 1980s certainly can attest to the hidden risks of supposedly safe blood products. What other undiscovered bloodborne pathogens lurk in our blood supply?
What the study clearly does not support, nor does Dr. Sims in any way advocate for this, is the sole use of vasopressin in the bleeding patient (see the EAST Traumacast were we discuss these ideas HERE. Blood products are the first-line and most important treatment in this scenario. But this study demonstrates that blood is perhaps not the only actor in this drama. Vasopressin may act in a supporting and complementary fashion. Further studies with more power to show survival advantage are needed, and in fact, demanded by the results of this trial.
Bottom Line: will I reach for the vasopressin next time I run the MTP on a bleeding trauma patient? I’m definitely considering it….
References:
- Gamper et al. Vasopressors for hypotensive shock. Cochrane Database Syst Rev. Feb 2016. PMID: 26878401
- P. Quenot et al. The epidemiology of septic shock in French intensive care units: the prospective multicenter cohort EPISS study. Crit Care. Apr 2013. PMID: 23561510
- De Backer et al. Comparison of dopamine and norepinephrine in the treatment of shock. N. Engl. J. Med. Mar 2010. PMID: 20200382
- Scott Weingart. EMCrit Podcast 205; Push-Dose Pressors Update. EMCrit Blog. Published on August 7th 2017.
- Thibonnier et al. Signal transduction pathways of the human V1-vascular, V2-renal, V3-pituitary vasopressin and oxytocin receptors. Prog. Brain Res. 1998. PMID: 10074787
- L. Holmes et al. The effects of vasopressin on hemodynamics and renal function in severe septic shock: a case series. Intensive Care Med. Aug 2001. PMID: 11511958
- Rhodes et al. Surviving Sepsis Campaign: International Guidelines for Management of Sepsis and Septic Shock. Critical Care Medicine. Mar 2017. PMID: 28098591
- Bouglé et al. Resuscitative strategies in traumatic hemorrhagic shock. Ann Intensive Care. Jan 2013. PMID: 23311726
- Beloncle et al. Does vasopressor therapy have an indication in hemorrhagic shock?. Ann Intensive Care. May 2013. PMID: 23697682
- L. Sperry et al. Early use of vasopressors after injury: caution before constriction. J Trauma. Jan 2008. PMID: 18188092
- W. Landry et al. Vasopressin Deficiency Contributes to the Vasodilation of Septic Shock. Circulation. Mar 1997. PMID: 9054839
- M. Cohn et al. Characterizing vasopressin and other vasoactive mediators released during resuscitation of trauma patients. J Trauma Acute Care Surg. Oct 2013. PMID: 24064875
- A. Sims et al. Arginine vasopressin, copeptin, and the development of relative AVP deficiency in hemorrhagic shock. Am. J. Surg. Oct 2017. PMID: 28716309
- Morales et al. Reversal by Vasopressin of Intractable Hypotension in the Late Phase of Hemorrhagic Shock. Circulation. Jul 1999. PMID: 10411844.
- D. Williams et al. Pressor effect of arginine vasopressin in progressive autonomic failure. Clin. Sci. Aug 1986. PMID: 3720194
- Sanui et al. Effects of arginine vasopressin during resuscitation from hemorrhagic hypotension after traumatic brain injury. Crit. Care Med. Feb 2006. PMID: 16424725
- A. Sims et al. Effect of Low-Dose Supplementation of Arginine Vasopressin on Need for Blood Product Transfusions in Patients With Trauma and Hemorrhagic Shock: A Randomized Clinical Trial. JAMA Surg. Aug 2019. PMID: 31461138
- Turan et al. Morbidity and mortality after massive transfusion in patients undergoing non-cardiac surgery. Can J Anesth. Aug 2013. PMID: 23609882
- Goel et al. Association of Perioperative Red Blood Cell Transfusions With Venous Thromboembolism in a North American Registry. JAMA Surg. Sep 2018. PMID: 29898202
For More Thoughts on This Checkout:
- St. Emlyn’s: AVP in Haemorrhagic Shock
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post AVERT-Shock: Vasopressin for Acute Hemorrhage? appeared first on REBEL EM - Emergency Medicine Blog.
