 Background Information: With rising cases, an increasing death toll, and a significant strain on hospital systems globally, the COVID-19 pandemic seemed to have no end in sight. The aggressive pursuit of a vaccine has led to multiple clinical trials starting before the end of this year. In fact, there are 48 vaccines under clinical evaluation and 11 of these are currently being evaluated in phase 3 clinical efficacy trials.1 Among those includes, the replication-deficient chimpanzee adenoviral vector developed at Oxford University (ChAdOx1). Following the initiation of a phase 1 clinical trial in the UK (COV001), three additional randomized controlled trials were initiated across the UK (COV002), Brazil (COV003) and South Africa (COV005). Upon completion of enrollment, the authors of the following paper sought to perform a combined interim analysis of the four trials to assess ChAdOx1’s efficacy and safety
Background Information: With rising cases, an increasing death toll, and a significant strain on hospital systems globally, the COVID-19 pandemic seemed to have no end in sight. The aggressive pursuit of a vaccine has led to multiple clinical trials starting before the end of this year. In fact, there are 48 vaccines under clinical evaluation and 11 of these are currently being evaluated in phase 3 clinical efficacy trials.1 Among those includes, the replication-deficient chimpanzee adenoviral vector developed at Oxford University (ChAdOx1). Following the initiation of a phase 1 clinical trial in the UK (COV001), three additional randomized controlled trials were initiated across the UK (COV002), Brazil (COV003) and South Africa (COV005). Upon completion of enrollment, the authors of the following paper sought to perform a combined interim analysis of the four trials to assess ChAdOx1’s efficacy and safety
Paper: Voysey M et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. Dec 2020 Epub ahead of print. PMID: 33306989
We have laid out each of the individual trials below so that you know who, where, and what was included in each of them. If you don’t have interest in that information jump down to the “Interim Analysis of All Study Data” section for the pooled results.
COV001 Study in UK
What They Did:
- Ongoing single-blinded (phase 1/2) clinical trial in five sites in the UK which began on April 23rd 2020
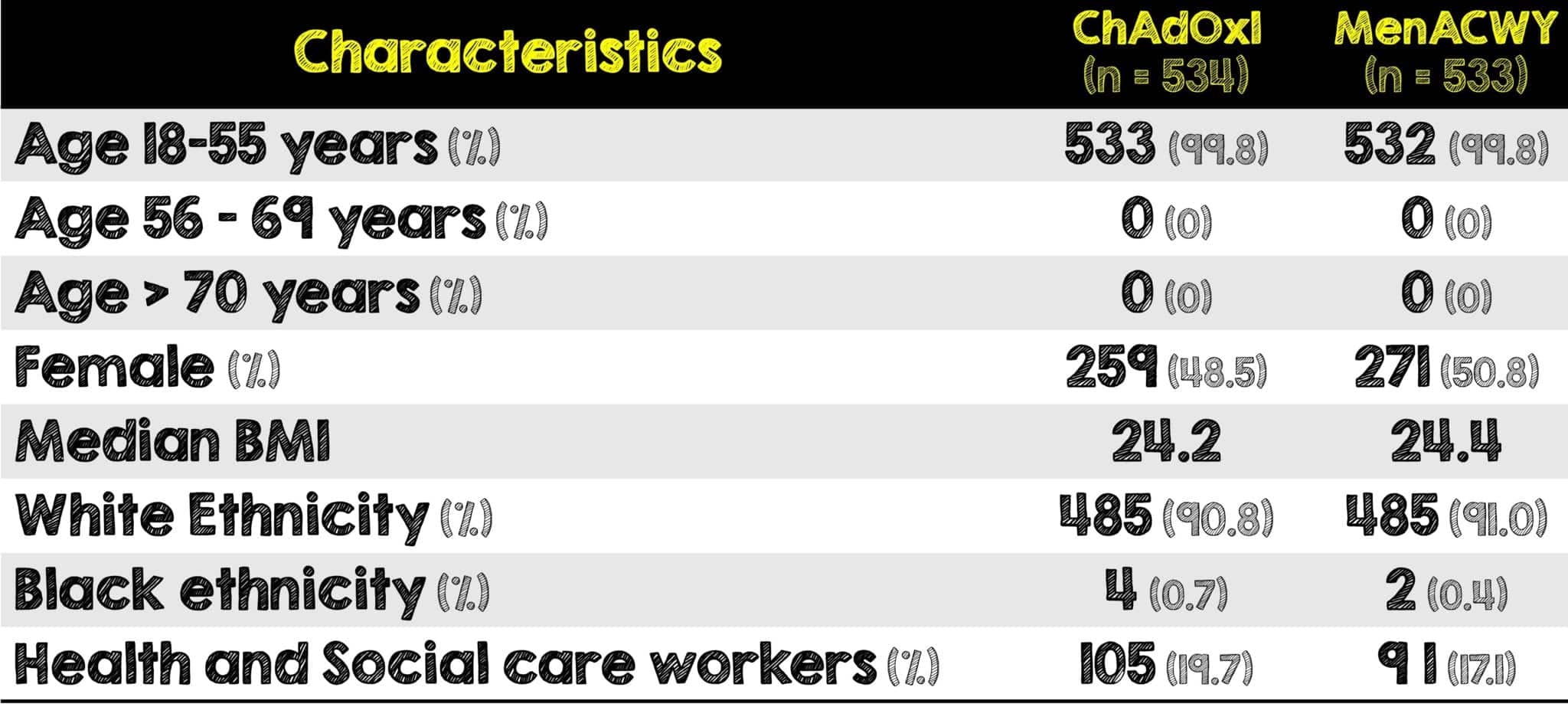
- Enrolled 1077 healthy volunteers aged 18 – 55 years
- Defined healthy as having no pre-existing conditions
- Participants were randomly assigned to either the control or experimental group:
- Experimental received the ChAdOx1 nCOVID-19 at a standard dose of 5×10^10 viral particles
- Control received the multi-group Meningococcal vaccine (MenACWY)
- Study was originally planned as a single-dose trial however the protocol was modified to a two-dose regime on July 30th 2020. 88 participants remain recipients of a single dose
- The outcome was to assess the efficacy and safety of ChAdOx1 Vaccine against the nCOVID-19 virus
- Participants will be followed 364 days after the last dose of the vaccine is administered
- All participants were given an emergency 24-hour telephone number to contact the on-call study physician for the duration of the study to report any illness
Inclusion Criteria:
- Healthy volunteers aged 18-55 years old
- Able and willing to comply with all study requirements some of which include:
- Refraining from blood donation during course of study
- For females, willingness to practice continuous effective contraception during study and a negative pregnancy test on day of screening and vaccination
- Not relying on public transportation or taxis for follow-up appointments
Exclusion Criteria:
- Volunteers under the age of 18 and over the age of 55
- Prior receipt of any vaccines (licensed or investigational) < 30 days before enrolment
- Have received immunoglobulins and/or any blood products within three months preceding vaccine administration
- Any confirmed or suspected immunosuppressive or immunodeficient state including HIV
- Use of any immunosuppressant medication within the past 6 months except topical or short-term oral steroids
- Any autoimmune condition except mild psoriasis, well-controlled autoimmune thyroid disease, vitiligo or stable celiac disease not requiring any immunomodulatory therapy
- History of allergic disease or reactions likely to be exacerbated by the vaccine
- Any history of angioedema
- Those with prespecified pre-existing conditions
- Pregnant or nursing patients
Outcomes:
Primary:
- Virologically confirmed symptomatic cases of COVID-19
- Occurrence of serious adverse events throughout study duration
Secondary:
- Local or systemic signs and symptoms of vaccine reaction within 7 days of administration
- Occurrence of adverse events for 28 days following vaccine administration
- Hospital and ICU admissions associated with COVID-19
- Deaths associated with COVID-19
- Severe COVID-19 disease (defined according to prespecified clinical severity scales ie. NEWS-2)
- Seroconversion against non-spike SARS-CoV-2 antigens
COV002 Study in UK
What They Did:
- Ongoing single-blinded phase 2/3 clinical trial which began on May 28th 2020 across 19 study sites in England, Wales and Scotland.
- Specifically, targeted working professionals with highest possible exposure to SARS-CoV-2 such as health care works and those in social care settings
- Broadened patient population to include HIV positive adults and healthy children
- Low doses were initially being administered. Following further review and identification of unexpected spectrophotometry assay interference, the protocol was changed to a standard dose regimen on June 5th 2020.
- As a result, the experimental group was divided into the following two distinct groups in addition to control group:
- LD/SD experimental group : Low dose (2.2×10^10 viral particles) as first dose plus standard dose as second dose
- SD/SD experimental group: Standard dose as both first and second dose
- Control: Received the multi-group Meningococcal vaccine (MenACWY)
- Staggered enrollment of older age patients, first starting with those 18-55 years old, then those aged 56-69 years old and finally participants over 70 years old
- Similar to COV001, participants in the 18-55 year old cohort were originally planned as single dose efficacy cohorts until the protocol was modified on July 20th 2020 to offer a second dose
- Primary outcome to assess efficacy of ChAdOx1 COVID-19 vaccine in adults and children
- Participants will be followed for 364 days after the last dose except certain groups which will be followed 364 days after the first dose
- Participants were asked to provide a weekly self-administered nose and throat swab for NAAT testing from 1 week after first vaccination using kits provided by the UK Department of Health, these included step-by-step instructions and a link to a video demonstrating how to do the self-swab. They were directly informed of their results by text/email from the National Health Service
Inclusion Criteria:
- Healthy adults aged > 18 years
- Immunogenicity sub studies included the following:
- Healthy children aged 5 to 12 years old
- HIV positive adults aged 18 – 55 years old receiving antiretroviral therapy
- Remaining inclusion criteria same as COV001
Exclusion Criteria:
- Prior receipt of any vaccines (licensed or investigational) < 30 days before enrolment
- Current diagnosis of or treatment for cancer (except basal cell carcinoma of the skin and cervical carcinoma in situ)
- History of serious psychiatric condition likely to affect participation in the study
- Bleeding disorder or continuous use of anticoagulants
- Suspected or known current alcohol or drug dependency
- Have received immunoglobulins and/or any blood products within three months preceding vaccine administration
- Any confirmed or suspected immunosuppressive or immunodeficient state (excluding HIV) such as asplenia
- Use of any immunosuppressant medication within the past 6 months except topical or short-term oral steroids
- Any autoimmune condition except mild psoriasis, well-controlled autoimmune thyroid disease, vitiligo or stable celiac disease not requiring any immunomodulatory therapy
- History of allergic disease or reactions likely to be exacerbated by the vaccine
- Any history of angioedema
- Severe and/or uncontrolled cardiovascular, respiratory, neurological, liver, renal or endocrine disease
- Pregnant or nursing patients
Outcomes:
Primary
- Assess vaccine efficacy by measuring virologically confirmed symptomatic cases of COVID-19 in adults and children
- Assess vaccine safety by measuring occurrence of serious adverse events in adult and children
Secondary
- Same as COV001
COV003 Study in Brazil
What They Did:
- Ongoing single-blinded randomized phase 3 control trial at six sites across brazil that began on June 23rd 2020.
- The target population was healthcare workers aged 18 years or older with highest possible exposure to SARS-CoV-2 who had stable pre-existing health conditions
- Participants were divided into one of the following two groups
- Experimental: Two standard doses of ChAdOx1 nCOVID19 vaccine up to 12 weeks apart
- Control: MenACWY vaccine for first dose followed by placebo for the second dose up to 12 weeks apart
- Similar outcome as COV001 and COV002 in assessing vaccine efficacy and safety
- Again patients had a 24-hour direct line to the on-call study physician to report any adverse signs or symptoms. Patients will be followed for 365 days post-randomization to record any adverse events and episodes of virologically confirmed symptomatic COVID-19
- Like the previous studies, a protocol amendment was made to include the second booster shot following immunogenicity results from prior studies
Inclusion Criteria:
- Adult patients aged 18 years or older who are health professionals and/or at high-risk of exposure to COVID-19
- Remaining inclusion criteria same as COV001
Exclusion Criteria:
- Participation in trials of prophylactic drugs for COVID-19 during the course of the study
- Any confirmed or suspected immunosuppressive or immunodeficiency state, including HIV (regardless of treatment, CD4 count or viral load status), asplenia
- Severe recurrent infections and chronic use (more than 14 days) of immunosuppressive medication in the last 6 months except for topical or short-term oral steroids
- Remaining exclusion criteria were identical to the COV002 study
Outcomes:
Primary
- Assess vaccine efficacy by measuring virologically confirmed symptomatic cases of COVID-19 in healthy adults over the aged 18 years and older
- Assess vaccine safety by measuring occurrence of serious adverse events in healthy adults aged 18 years and older
Secondary
- Same as COV001 except did not specifically look at ICU admissions
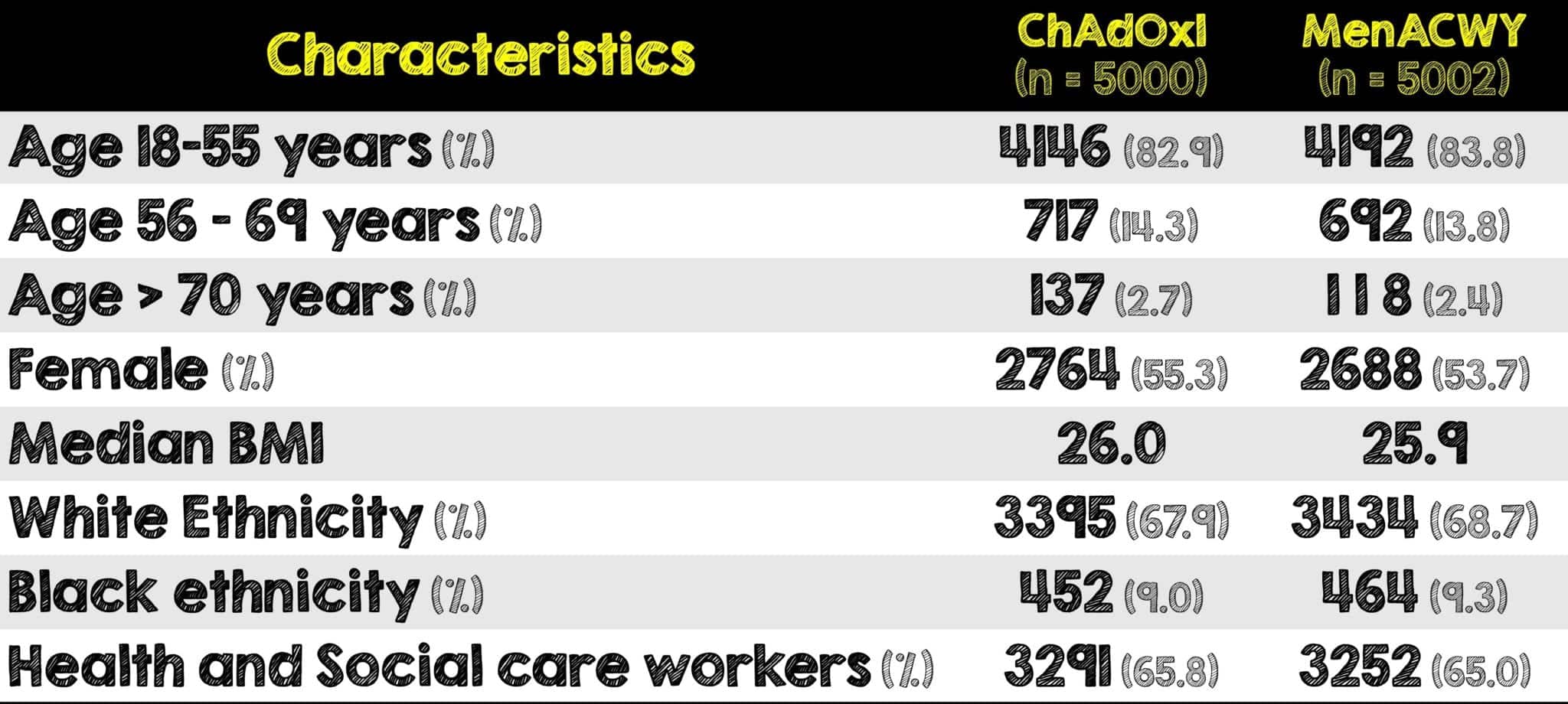
COV005 Study in South Africa
What They Did:
- Ongoing double-blind randomized phase 1/2 clinical trial at five sites in South Africa
- The target population was healthy adults aged 18 to 65 years living with and without HIV
- Participants were divided into one of four groups, three of which included people without HIV. Within those groups’ individuals received one of the following:
- Experimental: Two standard doses of ChAdOx1 nCOVID19 vaccine administered 4 weeks apart
- Control: Normal saline placebo administered 4 weeks apart
- Similar outcome assessing vaccine efficacy and safety as prior three clinical trials and most similar to second clinical trial (COV002 in the UK)
- Patients were given 24-hour direct line access to on-call study physician to report symptoms and were followed for 364 days following randomization
Inclusion Criteria:
- Healthy adults aged 18 – 65 years old
- Documented result of not being infected with HIV
- Those with HIV needed to have been on antiretroviral treatment for at least three months and HIV-1 viral load is <1,000 copies/mL within two weeks of randomization
- Remaining inclusion criteria as COV001
Exclusion Criteria:
- Any serious chronic illness requiring hospital specialist supervision
- Chronic respiratory diseases including poorly controlled/unstable asthma
- Overweight (BMI > 40 kg/m2)
- Suspected or known IVDU in the last 5 years
- Any clinically significant abnormal finding on urinalysis
- New onset of fever, cough or SOB in the 30 days before screening/enrollment
- Any confirmed or suspected immunosuppressive or immunodeficient state (including HIV for the three non-HIV groups), asplenia, recurrent severe infections and chronic use of oral steroids in the past 6 months (topical steroids were allowed)
- Remaining exclusion criteria similar to COV001
Outcomes:
Primary
- Assess safety and efficacy of ChAdOx01 COVID-19 vaccine in healthy HIV-uninfected adults between the ages of 18 and 65 years old
Secondary
- Assess the immunogenicity of ChAdOx01 COVID-19 vaccine in healthy HIV-uninfected adults
- Remaining secondary outcomes were similar to previous trials
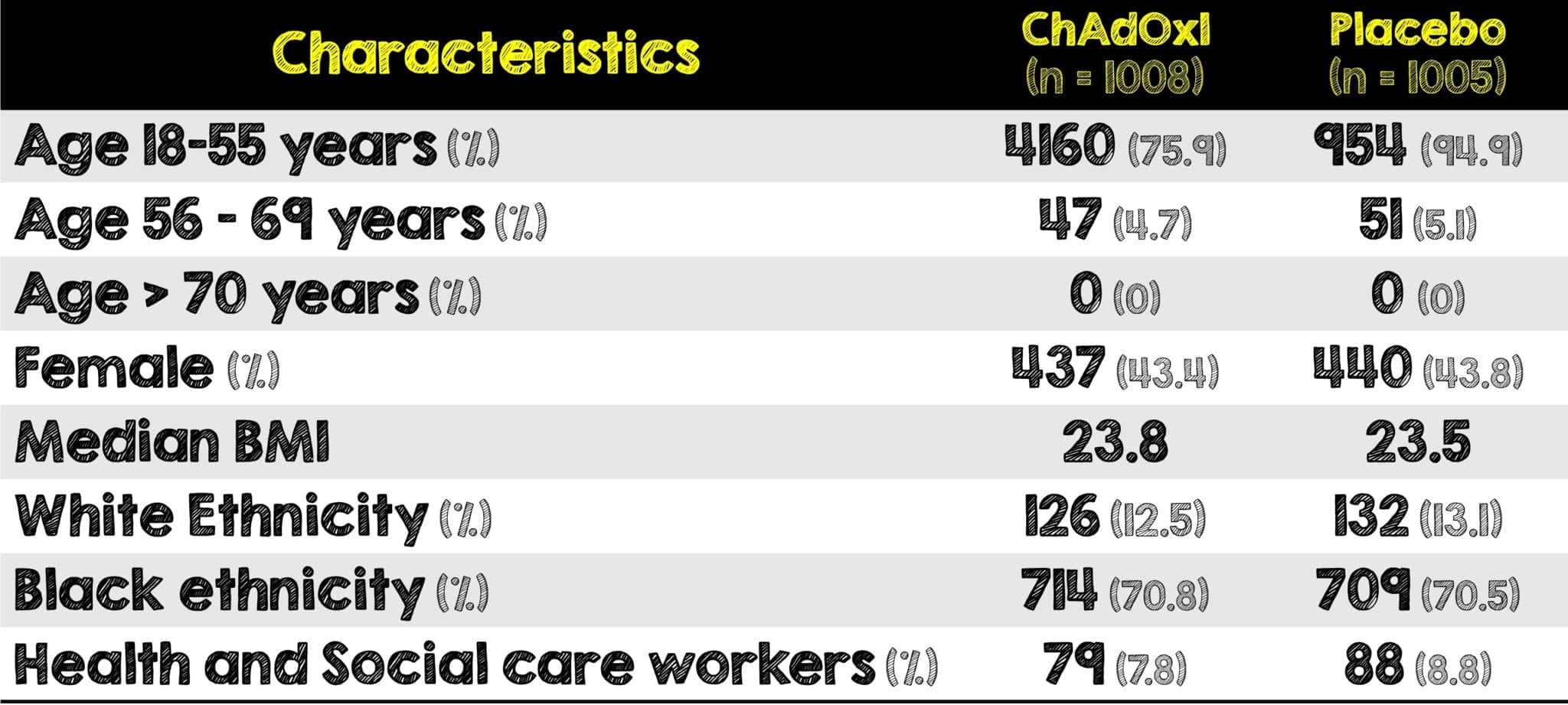
Interim Analysis of All Study Data
What They Did
- Pooled data from the above four trials together for greater precision of analysis of the efficacy and safety of the ChAdOx1 COVID Vaccine
- COV001 (phase 1/2; UK)
- COV002 (phase 2/3; UK)
- COV003 (phase 3; Brazil)
- COV005 (phase 1/2; South Africa)
- 3 of the studies were single blind and one is double blind (COV005)
- Randomized participants who received at least one dose in all the studies were included in the safety analysis
- Safety of the vaccine was assessed using data from all 4 studies
- Each study had to meet prespecified criteria of having at least five cases eligible for inclusion in the primary outcome before a study was included in efficacy analysis
- Neither COV001 or COV005 met these criteria and therefore are not included in the efficacy assessment
- Interim efficacy was assessed by prespecified global pooled analysis combining data from COV002 and COV003
- The authors defined outcomes specifically as the following:
- Symptomatic COVID-19: Any participant who had one of the many pre-specified list of five symptoms (ie. Fever, cough, shortness of breath, anosmia, ageusia)
- Non-Primary Symptomatic COVID-19: Any participant who had symptoms outside of the pre-specified list of five symptoms (ie. Diarrhea, malaise, headache, etc)
- Nucleic Acid Amplification Test (NAAT) = Participants who had one of the five pre-specified symptoms PLUS a positive COVID-19 nasal swab test
Exclusion Criteria:
- The following patients were excluded from the primary efficacy analysis:
- Seropositive patients at baseline or those that had no documented baseline result
- Patients not enrolled in an efficacy cohort or in non-randomized groups
- Patients not in a group that received two standard doses or a low-dose and standard-dose of the vaccine
- Vaccine administration errors
- Less than or equal to 14 days of follow-up accrued after the second dose
- Those who received a second dose but did not reach the 14 days post boost time point
- Those who chose not to receive a second dose or withdrew early
Critical Results:
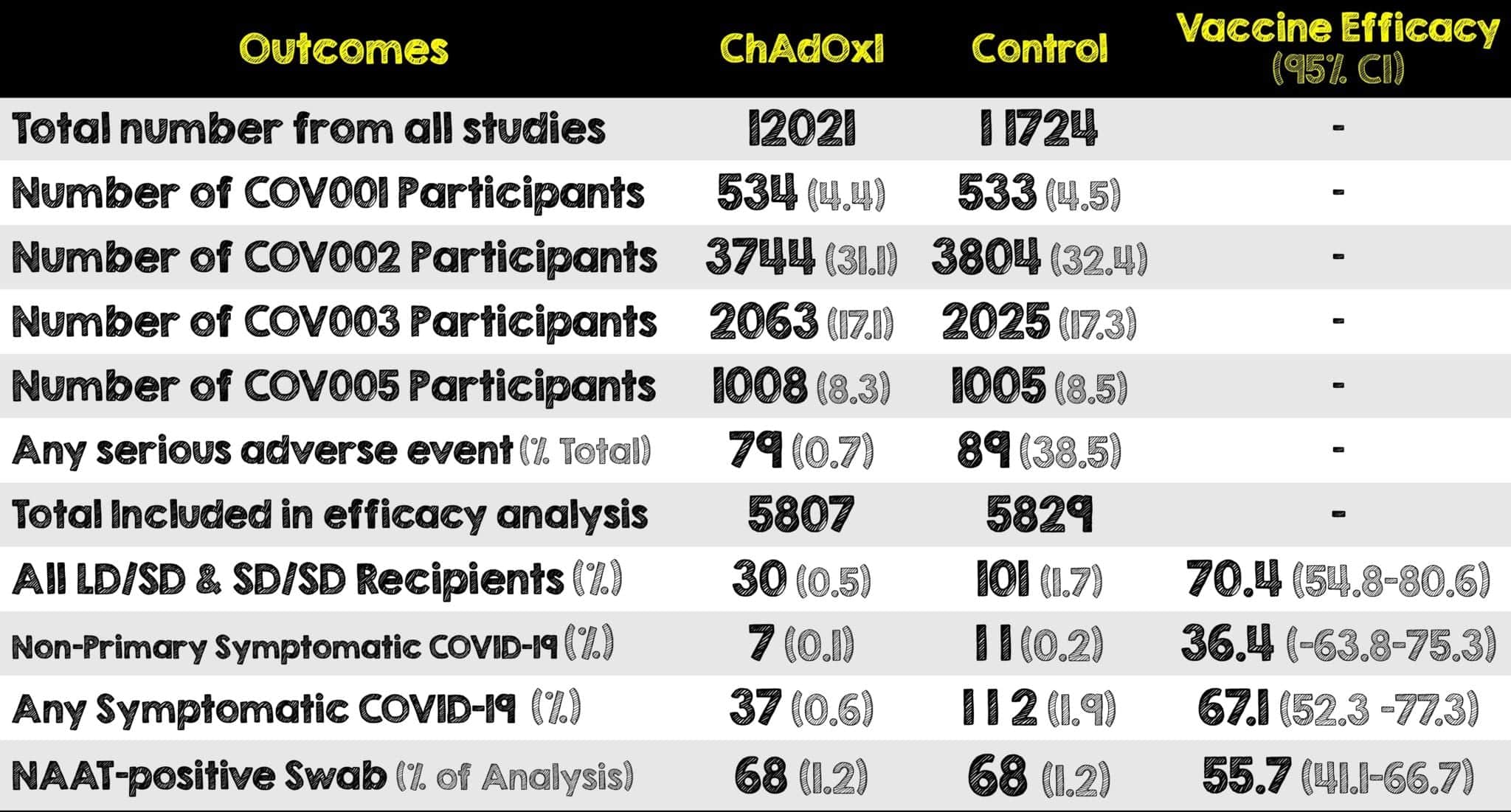
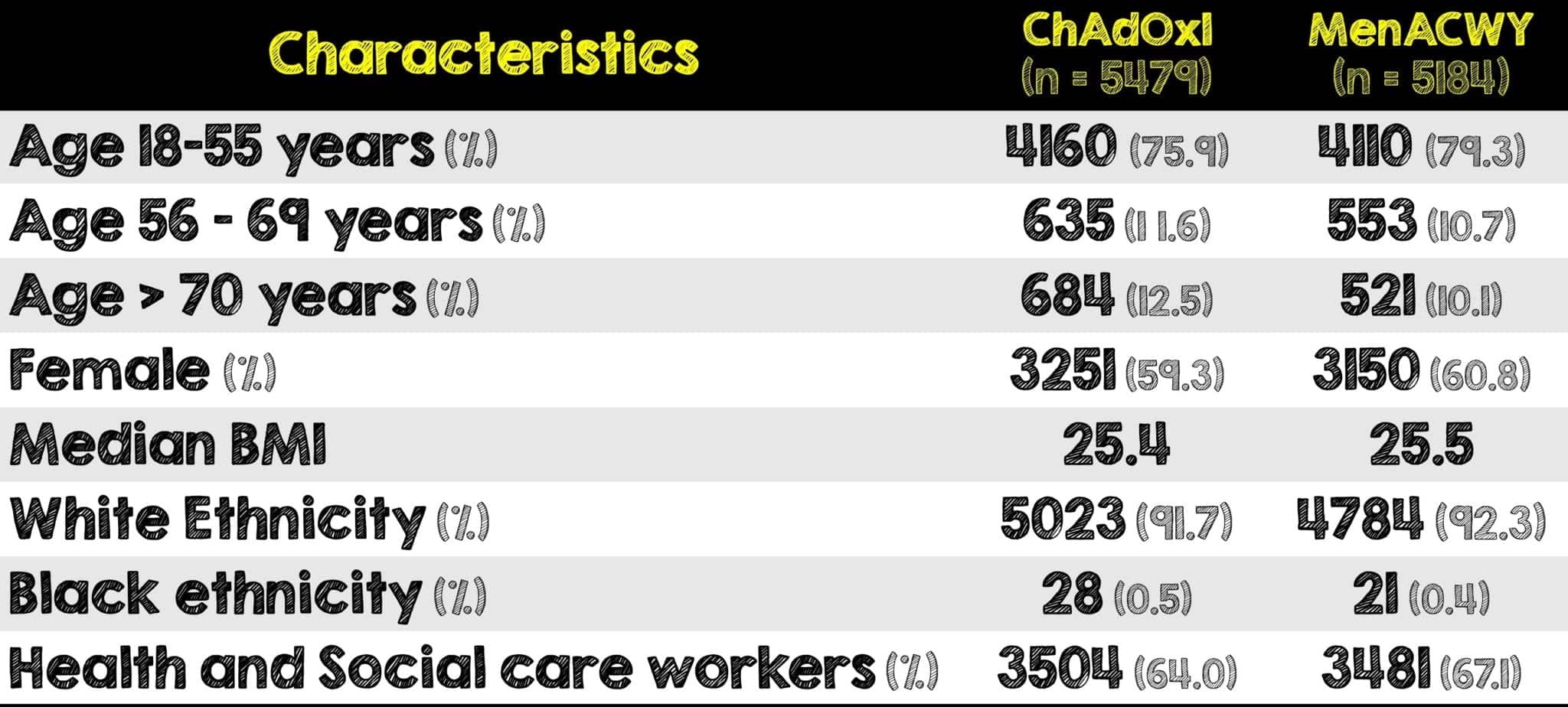
- 23848 participants were recruited and vaccinated across the four studies. 11636 participants in COV002 and COV003 met the inclusion criteria for the primary analysis.
- 5807 of those received two doses of ChAdOx1 nCov-19 Vaccine and the other 5829 received two doses of the control
- Majority of included patients were aged 18 – 55 years (≈88%)
- Those aged ≥56 years contributed to 12.2% of the total cohort
- There were 10 cases of hospitalization reported, all of which occurred in the control arm of the trial
Discussion:
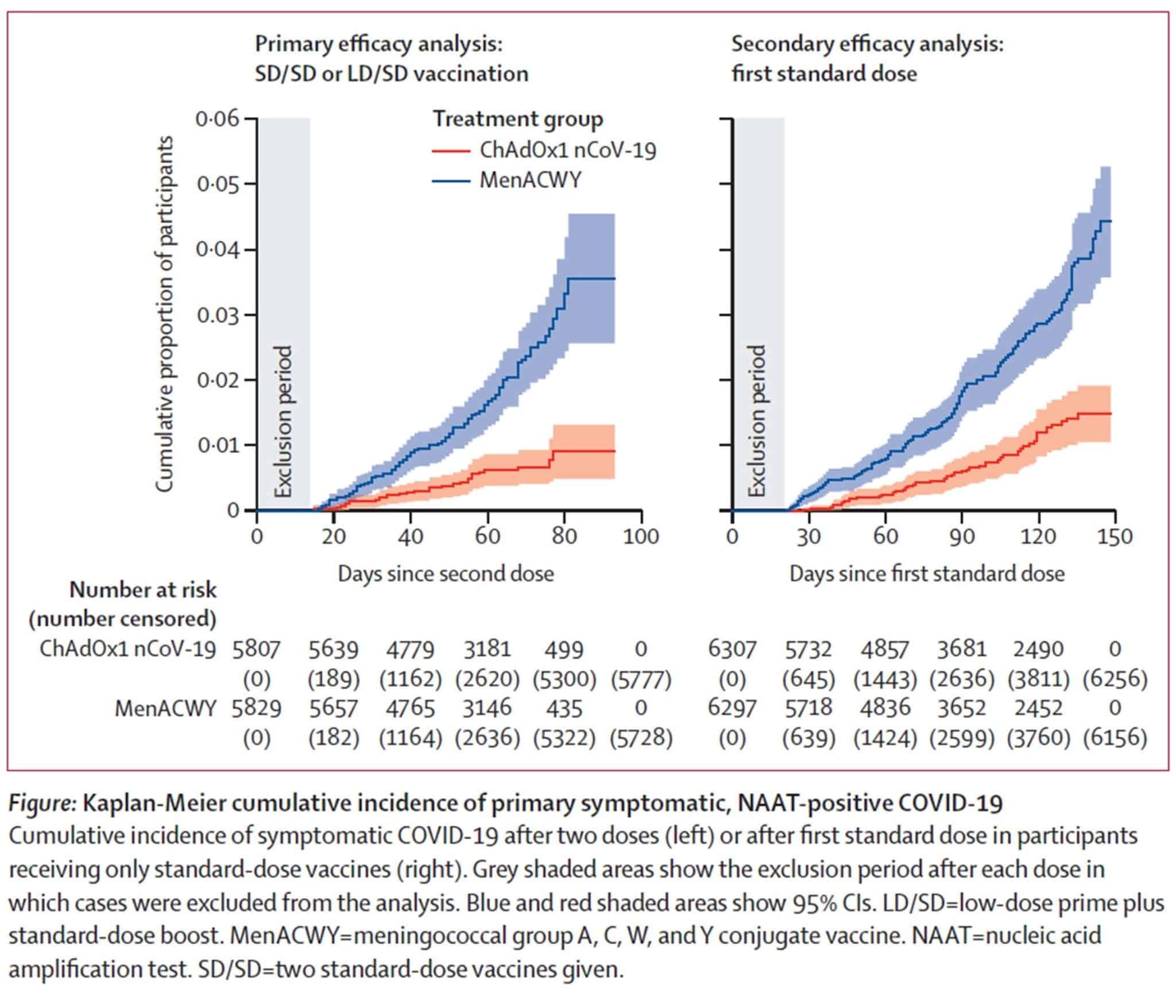
- Vaccine efficacy (combing dose groups) against the primary endpoint of COVID-19 positive more than 14 days after the second dose was 70.4% (95% CI: 54.8-80.6). Although efficacy is an important consideration, so are the pragmatics of delivery, community acceptance and longevity of effect
- The large sample size, randomization, inclusion of diverse sites and having similar results in Brazil and the UK lead to increased credibility of the study results
- It’s important to note that of all the four trials, COV001 and COV005 were excluded from efficacy assessment thus limiting the analysis to only participants in COV002 and COV003 who met inclusion criteria
- In participants who received two standard doses, efficacy against primary symptomatic COVID-19 was consistent in both the UK (60.3% efficacy) and Brazil (64.2% efficacy), indicating results are generalizable across two diverse settings with different timing for the booster dose (most participants in the UK received the booster dose >12 weeks after the 1st dose and most participants in Brazil received their second dose within 6 weeks of the first)
- Interestingly, the 90.0% efficacy was seen in those who received a low dose as the primer dose as opposed to a standard dose. This is intriguing but needs to be studied further as using a lower dose could substantially improve vaccine for distribution at a time of constrained supply. The wide Cis around these estimates show that further data is needed to confirm these preliminary findings
- HIV patients were excluded from interim analysis meaning the vaccine efficacy cannot be applied to that patient population. Also, older adults were recruited later in the trial meaning less time for cases to accrue and therefore the efficacy data on this patient population is limited by the small number of cases
- Less than 4% of the participants were older than 70 years old and no participants older than 55 years old received the mixed-dose regimen. Those with comorbidities were a minority with results for that subgroup not yet available
- All studies have completed enrollment of their respective efficacy cohorts and are in the follow-up phase. Additionally, pregnant patients, children, cancer patients, those with pre-specified conditions in the exclusion criteria were also not included, thus limiting the results of this interim analysis to a very narrow and specific patient population
- The US Food and Drug Administration guidelines indicate they would license a vaccine against COVID-19 that showed at least 50% efficacy and so has the WHO.2,3 While this vaccine was 70.4%, it’s significantly less than the other companies who’ve made their vaccine data and efficacy public. Both Moderna and Pfizer claim their vaccines have a 94.5% efficacy
- The four studies performed across three different countries enroll geographically and ethnically diverse patient populations. Specifically, in this analysis, those enrolled were predominantly younger, white, females and healthcare workers.
- The ChAdOx1 vaccine can be refrigerated at higher temperatures (2-8oC) compared to the other mRNA COVID-19 vaccines. This better facilitates global distribution and overall availability as the ultra-low temperature freezers required to store the other vaccines may be too expensive and impractical in many countries and other care settings such as nursing homes
- Furthermore, it is concerning that in the studies, one of the inclusion criteria was “reliable transportation”, explicitly stating individuals should not dependent on public transportation or taxis. This form of very obvious implicit bias targets individuals of color and those who are impoverished which again have been shown to be more disproportionately affected by severe COVID-19.4,5
- The authors claim that although there were many serious adverse events reported, there was no pattern of these events that provided a safety signal in the study. Three cases of transverse myelitis were initially reported as suspected serious adverse reactions. Following an independent clinical review of the two cases (one in the control and one in the vaccine arm), the authors report them as unlikely being related to the study interventions. The third case that also occurred in the vaccine arm, still has a possible relationship to the study interventions.
- Also note that all but one of the four trials are single-blinded and on-going studies. Therefore, as new information is revealed when the patients are followed-up, the results may significantly change from what is presented here
- Like any major study funded by a big pharmaceutical company, caution and skepticism are warranted when interpreting their results. This is especially the case in a high-stakes analysis such as this where three of the authors who directly contributed to the implementation and data collection are employees of AstraZeneca
- This vaccine data helps provide useful information; however, it does not tell us the duration of protection (ie. whether additional booster shots are required) or whether asymptomatic vaccinated individuals can still transmit the virus to others who have not been vaccinated. Public health measures such as washing hands, wearing a mask and social distancing still remain vitally important to limiting the spread of COVID-19
Author’s Conclusions:
- ChAdOx1 nCoV-19 has an acceptable safety profile and has been found to be efficacious against symptomatic COVID-19 in this interim analysis of ongoing clinical trials.
Our Conclusion:
- ChAdOx1 nCoV-19 has been found to be efficacious against symptomatic COVID-19 in this interim analysis and by governing regulatory body (FDA, WHO) standards. While this remains a much-needed intervention amidst a deadly pandemic, the methodology of this analysis and the release of other more efficacious vaccines at the current moment should incite healthcare workers towards those alternatives if a choice is presented. Lastly, regardless of which vaccine a person has received, public health measures such as washing hands, mask wearing, and social distancing should continue to be done.
Clinical Take Home Point:
- ChAdOx1 is an efficacious vaccine by FDA and WHO standards to a very narrow subset of patients. The 90.0% efficacy seen in those who received a low dose as the primer dose as opposed to a standard dose is intriguing but needs to be studied further as using a lower dose could substantially improve the amount of vaccine for distribution at a time of constrained supply as well as improve efficacy. This much needed vaccine should not yet eliminate effective public health measures that have been used to reduce the virus’ spread such as: mask wearing, hand washing and social distancing
REFERENCES:
- Voysey M et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. Dec 2020 Epub ahead of print. PMID: 33306989
- Center for Biologics Evaluation and Research. Development and licensure of vaccines to prevent COVID-19. June, 2020. Link here
- WHO. WHO target product profiles for COVID-19 vaccines. April 9, 2020. Link Here
- Richards-Belle A, et al. COVID-19 in critical care: epidemiology of the first epidemic wave across England, Wales and Northern Ireland. Intensive Care Med. 2020 Nov 2020. PMID: 33034689
- Sze S, et al. Ethnicity and clinical outcomes in COVID-19: A systematic review and meta-analysis. EClinicalMedicine. Nov 2020 PMID: 33200120
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srreziae)
The post COVID-19 Update: The ChAdOx1 (AstraZeneca) COVID-19 Vaccine appeared first on REBEL EM - Emergency Medicine Blog.