
 Background Information: The administration of alteplase (tPA) in acute ischemic stroke (AIS) continues to remain a highly debated topic. As hospital systems continue to undergo major changes to facilitate this controversial drug’s administration, more studies are coming out focusing on neuroimaging and how it plays a role in the time window of AIS. The WAKE-UP trial was one of the first studies to identify MRI patterns suggestive of a stroke in patient whose onset time was unknown.1,2 Over the past 10+ years, other studies have also attempted to identify the role of advanced neuroimaging guiding tPA administration for improved functional outcomes. The authors conducted a meta-analysis to test the hypothesis that tPA improves functional outcomes compared with placebo 4.5 – 9 hours after onset in AIS patients who received advanced neuroimaging. Before getting into the study, we need to better understand the terminology and different types of neuroimaging modalities available and how they play a role in strokes.
Background Information: The administration of alteplase (tPA) in acute ischemic stroke (AIS) continues to remain a highly debated topic. As hospital systems continue to undergo major changes to facilitate this controversial drug’s administration, more studies are coming out focusing on neuroimaging and how it plays a role in the time window of AIS. The WAKE-UP trial was one of the first studies to identify MRI patterns suggestive of a stroke in patient whose onset time was unknown.1,2 Over the past 10+ years, other studies have also attempted to identify the role of advanced neuroimaging guiding tPA administration for improved functional outcomes. The authors conducted a meta-analysis to test the hypothesis that tPA improves functional outcomes compared with placebo 4.5 – 9 hours after onset in AIS patients who received advanced neuroimaging. Before getting into the study, we need to better understand the terminology and different types of neuroimaging modalities available and how they play a role in strokes.
Terminology:
- Diffusion Tensor Imaging (DTI) = A tool for assessing organs that are primarily fibrous in their structure (i.e. Central nervous system and brain)
- Diffusion-weighted Imaging (DWI) = Combines functional information with anatomic information obtained from conventional MRIs to identify ischemic strokes. Uses DTI to produce significant detail of white matter integrity
- Perfusion-weighted Imaging (PWI) = When combined with DWI and angiography, can identify salvageable areas of ischemia.3
- Fluid Attenuated Inversion Recovery (FLAIR) = An advanced MRI sequence that removes signal from the CSF and allows for visualization of superficial brain lesions. Also allows for differentiation between ischemic and infarcted strokes. 4
- Perfusion-Diffusion Mismatch: An extremely time-dependent abnormality of volume between DWI and PWI which provides a practical and approximate measure of the tissue at risk in ischemic stroke.5
Clinical Question:
- Does intravenous alteplase improve functional outcomes compared with placebo in patients with ischemic stroke 4.5 – 9 hour after onset or wake-up stroke who were imaged with CT perfusion or perfusion-diffusion MRI?
What They Did:
- Systematic review and meta-analysis of individualized patient data for trials using Alteplase with placebo (0.9 mg/kg, max of 90 mg delivered as a 10% bolus and 90% infusion over 1 hour) published in English between January 1st 2006 and March 1st 2019 using the following search terms:
-
- “Stroke”
- “Randomized” or “Randomised”
- “Thrombolysis”
- “Alteplase” or “tPA”
- Authors also reviewed the reference list of previous systematic reviews of thrombolysis
- ClinicalTrial.gov was also searched for interventional studies of ischemic stroke
- The authors identified the following three studies which met the inclusion criteria: EXTEND, ECASS-4:EXTEND and EPITHET (see Table 1)
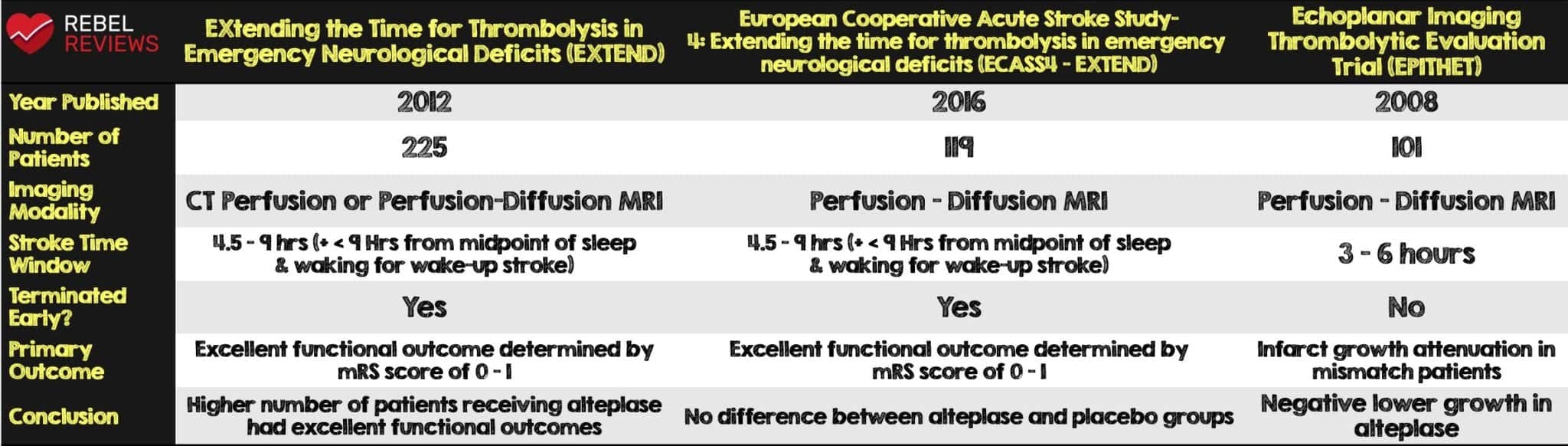
- The investigators from all three studies agreed to pool individual patient clinical and imaging data to be used for this meta-analysis
- The authors used the modified Rankin Score (mRS) to assess functional improvement, independence and outcomes (see Table 2)

Inclusion Criteria:
- Randomized
- Multicenter
- Double blind
- Age ≥ 18 years old
- 2 arm trials with 1:1 randomization of IV alteplase vs placebo
- Patients with >4.5 hours from onset or after last known well
- Acquired pre-treatment imaging with perfusion-diffusion MRI or CT perfusion
- Patients with available data on the following:
- Age
- Pretreatment National Institutes of Health Stroke Scale (NIHSS)
- mRS at 3 months
Exclusion Criteria:
- Any study where the patients did not receive CT perfusion or perfusion-diffusion MRI imaging
Outcomes:
Primary:
- Functional outcome determined by a mRS score 0 – 1 at 3 months
- Adjusted for pretreatment clinical severity using NIHSS and age
Secondary:
- Functional improvement, determined by a ≥1-point reduction in mRS score with categories 5 and 6 merged at 3 months (ordinal shift analysis)
- Functional independence, determined by a mRS score of 0 – 2 at 3 months
- Early neurologic improvement determined by a reduction of ≥ 8 points on the NIHSS or reaching NIHSS score 0 – 1 at 72 hours
- All secondary outcomes listed above were adjusted for pretreatment clinical severity using NIHSS and age
Safety:
- Symptomatic intracerebral hemorrhage defined as a blood clot occupying >30% of the infarcted territory with substantial mass effect within 36 hours of treatment
- Neurologic deterioration of ≥4 NIHSS points or death
Results:
- Three trials met eligibility criteria: EXTEND, ECASS4-EXTEND and EPITHET
- There were a total of 414 patients across all three trials
- 213 (51%) were assigned to receive alteplase
- 201 (49%) received the placebo
- Median time from last known well to treatment time in patients with wake-up stroke was 10 hours and 42 minutes (IQR 8 hours 40 minutes – 12 hours and 20 minutes)
- The median perfusion mismatch was 47 mL (IQR 17 – 85) with a median volume of critical hypoperfusion of 64 mL (30 – 109) and relatively small core of median volume 8 mL (0 – 22) (ie. Small core infarct with large ischemic penumbra).
- 211 patients in the alteplase and 199 patients in the placebo group had mRS data available at three months and were included in primary outcome results
- Perfusion mismatch status using an automated software was possible in 405 patients and 304 patients had a perfusion mismatch
- 152 (73%) of 207 in the alteplase group
- 152 (77%) of 198 in the placebo group
- Overall of the 403 patients with assessable angiographic imaging, 246 (61%) had large vessel occlusion that would potentially be amenable to endovascular thrombectomy
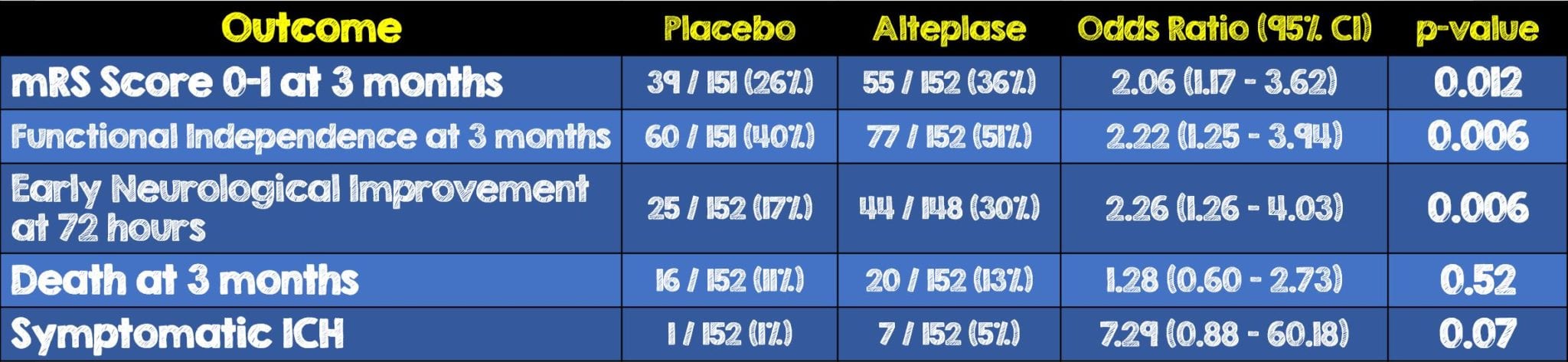
- 76 (36%) of the 211 in the alteplase group achieved the primary outcome compared with 58 (29%) of the 199 in the placebo group
Critical Results:

- 10 (5%) of the 213 patients given alteplase had symptomatic intracerebral hemorrhage compared to the one patient out of 201 in the placebo group
- No significant differences were identified in mortality between the 29/213 (14%) patients who received alteplase and the 18/201 (9%) patients in the placebo group (OR 1.55, 95% CI 0.81-2.96, p = 0.19).
- No significant differences were identified in the proportion of patients with combined mRS scores of 5 or 6. 48 (23%) of 211 in the alteplase group vs 41 (21%) of the 199 patients in the placebo group (adjusted OR 1.03, 95% CI 0.62-1.74, p = 0.897)
- Fatal intracerebral hemorrhage occurred in 2.3% of patients in this analysis
- Among a subcohort of the larger patient population, 303 patients with automated perfusion mismatch who had available mRS scores at 3 months, 55 (36%) of the 152 patients in the alteplase group achieved excellent functional outcome compared to the 39 (26%) of the 151 in the placebo group (OR 2.06, 95% CI 1.17-3.62, p = 0.012)
- Among the patients with automated perfusion mismatch, significant improvements in all secondary outcomes were observed in the alteplase group compared to the placebo group.
- In patients without mismatch, there were no significant differences between the two groups
- Automated perfusion mismatch selection did not seem to alter the risk of symptomatic intracerebral hemorrhage in the alteplase group compared with all patients
Strengths:
- Investigators and management committees from all three studies had a statistician pool together all their data for the purposes of this meta-analysis (Management committees of all included trials agreed to share individual patient level data for the purposes of this meta-analysis)
- Study authors utilized a wide date range, many search terms and even searched current clinical trials for interventional studies of ischemic stroke to make this meta-analysis as comprehensive as possible
- Central adjudication of perfusion mismatch status using automated RAPID software was possible in 405 patients (raw imaging data were not available for six patients in the alteplase group and three patients in the placebo group)
- Had two reviewers independently assess articles for inclusion
- Attempted to sub-divide the modified Rankin Scale into definitions of practical outcomes relating to a patient’s function and independence
- Accounted for between trial variance by using a mixed-effects logistic regression model which were incorporated as random effects in all models
- Utilized a stroke neurologist with extensive neuroimaging analysis experience who was masked to treatment allocation and all other clinical information to visually verify and remove artifacts from imaging
- Boehringer Ingelheim provided investigational study products free of charge, however had no input into the study design, writing of the protocol, data collection, data analysis or data interpretation.
Limitations:
- Two of the three studies (EXTEND and ECASS4-EXTEND) were terminated early due to external factors. This limits the overall sample size.
- All patients were recruited in Australia, New Zealand, Europe and Asia which have different healthcare systems when compared to the United Stated. This may limit the results of this study to a United States healthcare system
- Perfusion imaging is not available in many regions and hospitals overall thus limiting this study’s external validity and reproducibility
- Study aimed at a very narrow subset of institutions which don’t have endovascular thrombectomy, can administer thrombolysis, and have advanced diffusion and perfusion-weighted neuroimaging.
- The authors report that the commercially available automated software they used is costly and then still requires physician oversight. This restricts the generalizability of the study to more resource-rich institutions
- This meta-analysis was performed before evidence supporting endovascular thrombectomy was published. 97% of the patients (403 of 414) were recruited before this guideline change was made in February 2018, recommending thrombectomy in the 6 – 24 hour window. As a result, consideration of thrombectomy was an exclusion criterion in the three trials included
- NIHSS baseline score not equal in both groups: median NIHSS score 12 [IQR 7–17] vs 10 [6–16] but not statistically significant
- The analysis was underpowered to detect a statistical interaction between mismatch status and treatment effect. The magnitude of difference in point estimates suggests that the effect is driven by patients meeting perfusion mismatch criteria.
Discussion:
- This is the first meta-analysis review combining several studies which used advanced neuroimaging to determine the benefit of tPA administration in a stroke time frame of 4.5 to 9 hours and wake up strokes.
- The authors report their literature review started with the year 2006 because it was the year when thrombolysis in patients with perfusion diffusion mismatch was described in the non-randomized DEFUSE study
- While the modified Rankin Scale is a widely accepted scoring system used to categorize various levels of disability, there is still some significant gray area in applying it generally to all patients. “Some help” or “assistance” varies in its meaning and practical application to patients and their daily activities.
- The concept of mismatch can not be understated here, if a patient’s infarct core is big and the ischemic portion is small then they will likely have a small chance of benefit. The opposite is true, if the infarct core is small and their ischemic portion is large, there is a better chance of benefit. The rate of symptomatic intracerebral hemorrhage remains the same regardless of mismatch or not.
- Patients who met automated mismatch criteria had a significant benefit of alteplase in terms of functional outcome and independence and neurological improvement. This benefit did not reach statistical significance in patients without perfusion mismatch. Furthermore, no statistically significant interaction was identified between mismatch status and treatment effect.
- The confidence intervals in this analysis were very wide and close to crossing zero. It is unclear whether the results would remain statistically significant if this had a larger patient population.
- When compared to previous studies where alteplase was administered within the 0 – 3 hour stroke-onset window the adjusted OR for achieving an mRS score of 0 – 1 among all patients was 1.75 (95% CI, 1.35 – 2.27), for alteplase administered in the 3 – 4.5 hour stroke onset window 1.26 (95% CI, 1.05 – 1.51). 6 In this analysis the adjusted OR was 1.86 (95% CI 1.15 – 2.99) among all patients and 2.06 (95% CI, 1.17 – 3.62) for patients with automated perfusion mismatch.
- The treatment effect with alteplase was numerically lower than that observed with endovascular thrombectomy (18% Difference in mRS score 0 – 1).7,8
- Over half of the patients had large vessel occlusion that would have been amenable to systemic alteplase. As mentioned earlier, 97% of the patients were recruited before the February 2018 guideline changes for thrombectomy were made and thus the consideration of thrombectomy was an exclusion criterion across all three studies. In current practice, 61% of the patients in this analysis would now be eligible for thrombectomy due to the presence of a proximal large vessel occlusion. The combined used of thrombolysis and endovascular thrombectomy in an extended time window is currently being investigated in an active, ongoing trial (NCT03785678).
Author’s Conclusions:
- Patients with ischaemic stroke 4.5 – 9 h from stroke onset or wake-up stroke with salvageable brain tissue who were treated with alteplase achieved better functional outcomes than did patients given placebo. The rate of symptomatic intracerebral haemorrhage was higher with alteplase, but this increase did not negate the overall net benefit of thrombolysis.
Our Conclusion:
- For patients without large vessel occlusion, this data pushes to expand the number of patients with AIS who may benefit from systemic alteplase. (ie. 4.5 – 9 hour window + wake up strokes). Functional independence is a subjective outcome that varies from patient to patient and is difficult to categorize with a single scoring system. Furthermore, not all institutions have access to the advanced neuroimaging modalities used in this study which limits its utility in more rural and community facilities. Lastly the overall application of this study may be further limited by the very select population of AIS patients. These AIS patients must have a small core infarct and larger ischemic penumbra to gain potential benefit, all while the risk of intracerebral hemorrhage remains the same as previous studies (i.e. 5-6%).
Clinical Bottom Line:
- We should no longer be using the paradigm “Time is Brain” in AIS. The advancement of neuroimaging and perfusion-based imaging in an extended stroke window is the future of stroke care by helping identify a limited population of patients that would benefit from systemic alteplase vs those with no chance to benefit at all. The difficult decision to administer systemic alteplase should be based on the individual patient’s overall clinical picture and come following an in-depth shared-decision making discussion with the patient, and/or their family, explicitly stating the benefits and risks, including those of death and further hemorrhage.
REFERENCES:
- Campbell BCV, et al. Extending thrombolysis to 4·5-9 h and wake-up stroke using perfusion imaging: a systematic review and meta-analysis of individual patient data. Lancet. 2019. PMID: 31128925
- Thomalla G, et al. MRI-guided thrombolysis for stroke with unknown time of onset. N Engl J Med 2018; PMID: 29766770
- Baliyan V, et al. Diffusion weighted imaging: Technique and applications. World J Radiol. 2016; PMID: 27721941
- Bakshi R, et-al. Fluid-attenuated inversion recovery magnetic resonance imaging detects cortical and juxtacortical multiple sclerosis lesions. Arch. Neurol. 2001; PMID: 11346369
- Chen F, Ni YC. Magnetic resonance diffusion-perfusion mismatch in acute ischemic stroke: An update. World J Radiol. 2012; PMID: 22468186
- Emberson J, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. Lancet 2014; PMID: 25106063
- Nogueira RG, et al. Thrombectomy 6 to 24 hours after stroke with a mismatch between deficit and infarct. N Engl J Med 2018; PMID: 29129157
- Albers GW, et al. Thrombectomy for stroke at 6 to 16 hours with selection by perfusion imaging. N Engl J Med 2018; PMID: 29364767
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Extending Systemic Thrombolysis to 4.5 – 9 Hours and Wake-Up Strokes Using Perfusion Imaging: A Meta-Analysis appeared first on REBEL EM - Emergency Medicine Blog.
