 Background: Chest pain is the second most common reason for presentation to the emergency department (ED) and accounts for nearly 6.5 million visits each year. [1] Management often requires the utilization of clinical gestalt, laboratory analysis, diagnostic imaging, and clinical prediction rules.
Background: Chest pain is the second most common reason for presentation to the emergency department (ED) and accounts for nearly 6.5 million visits each year. [1] Management often requires the utilization of clinical gestalt, laboratory analysis, diagnostic imaging, and clinical prediction rules.
Clinical decision instruments (CDIs) are “clinical tools that quantify the individual contributions that various components of the medical history, physical examination, and basic laboratory results make toward the diagnosis, prognosis, or likely response to treatment in an individual patient.”[2] We use CDIs to assist with complex decisions.
This paper sought to validate the Beckman – Coulter Access high sensitivity troponin (hs-TnI) assay with 5 accelerated diagnostic pathways for chest pain.
- HEART score [Link is HERE]
- m-ADAPT [Link is HERE]
- EDACS pathway [Link is HERE]
- Vancouver chest pain rule [Link is HERE]
- No Objective Testing (NOT) Rule
Paper: Greenslade JH et al. Diagnostic Accuracy of a New High-Sensitivity Troponin I Assay and Five Accelerated Diagnostic Pathways for Ruling Out Acute Myocardial Infarction and Acute Coronary Syndrome. Ann Emerg Med. 2018. PMID: 29248334
Clinical Question: Do diagnostic chest pain pathways commonly used to rule out AMI and/or ACS remain effective when used with a newer high sensitivity troponin assay (Access hs-TnI)?
What They Did:
-
Data from two prior studies at tertiary care hospitals in Australia conducted to develop and validate chest pain protocols in the ED
-
The Australian cohort of the ADAPT study [Link is HERE]
- Prospective observational study of 986 adult patients presenting to the ED with chest pain
-
The Improved Assessment of Chest Pain trial [Link is HERE]
- Interventional trial including 1366 adult patients
- Studies had the same inclusion criteria, same variables, and procedures for data collection, and had data available for the Access hs-TnI
- The validation of clinical decision rules for the evaluation of ACS was part of the protocol for both studies.
-
The Australian cohort of the ADAPT study [Link is HERE]
- ECG and troponin samples were taken at 0 and 2 hours
- Samples analyzed using Access hs-TnI assay in a blinded fashion
-
99th percentile is used for the cutoffs for troponin positivity
-
The Access hs-TnI assay has a 99th percentile cutoff of 17.5 ng/L
- 99th percentile cut point for women 11.6 ng/L
- 99th percentile cut point for men 19.8 ng/L
-
The Access hs-TnI assay has a 99th percentile cutoff of 17.5 ng/L
- Retrospectively classified as “low risk” or “not” according to m-ADAPT, EDACS, HEART, Vancouver Chest Pain Rule, and No Objective Testing Rule score pathways
- All pathways except for HEART were calculated as part of the original dervication study
-
HEART Pathway retrospectively calculated
-
- 2-hour troponin used instead of 3-hour troponin in previous validation study
-
-
30 day follow up phone call and medical record review
-
Information obtained from patients and hospital databases
- Cardiac events
- Cardiac investigations: stress test, echo, CT Angio, or PCI
- Contact with any health care provider during the 30-day period
- All information was verified through contact with health care providers and copies of original medical records
- No loss to follow up
-
Information obtained from patients and hospital databases
Inclusion:
- ≥ 18 years old with ≥ 5 mins of chest pain consistent with ACS
- Enrolled from 8 am to 5 pm
- Pts were undergoing an ACS workup
-
Pain due to ACS:
- Acute chest, epigastric, neck, jaw or arm pain or discomfort or pressure without clear noncardiac source.
Exclusion Criteria:
- The patient met STEMI criteria
- Clear alternative cause for the suspected symptoms other than ACS
- Participants unwilling or unable to provide informed consent
- Recruitment deemed inappropriate (eg. palliative patients)
- Pregnant patients
- Participants recruited to study in the past 45 days
- Transferred from another hospital
- Could not be contacted after discharge (eg. homeless)
Primary Outcomes:
-
30-day AMI used to evaluate m-ADAPT and EDACS
- Death from a cardiac cause
- NSTEMI
- STEMI
- Emergency revascularization on admission or within the 30-day follow up
-
30-day ACS used to evaluate HEART score, Vancouver Chest Pain Rule & No Objective Testing Rule
- Death from a cardiac cause
- NSTEMI
- STEMI
- Emergency or unplanned revascularization on admission or within the 30-day follow up
- Unstable angina pectoris on admission or within the 30-day follow up
Results
-
1,811 patients
- 96 patients (5.3%) diagnosed with 30-day AMI
- 139 patients (7.7%) diagnosed with 30 day ACS
-
1,660 patients with hs-TnI below 99th percentile at presentation
- 23 of these had AMI, sensitivity 76% (95% CI 66.3% – 84.2%)
- 30 patients had elevated hs-TnI at 2 hours
-
1630 patients with hs-TnI below 99th percentile at presentation and 2 hours
- 10 of these patients diagnosed with 30-day AMI, sensitivity 89.6% (95% CI 81.7% – 94.9%)
-
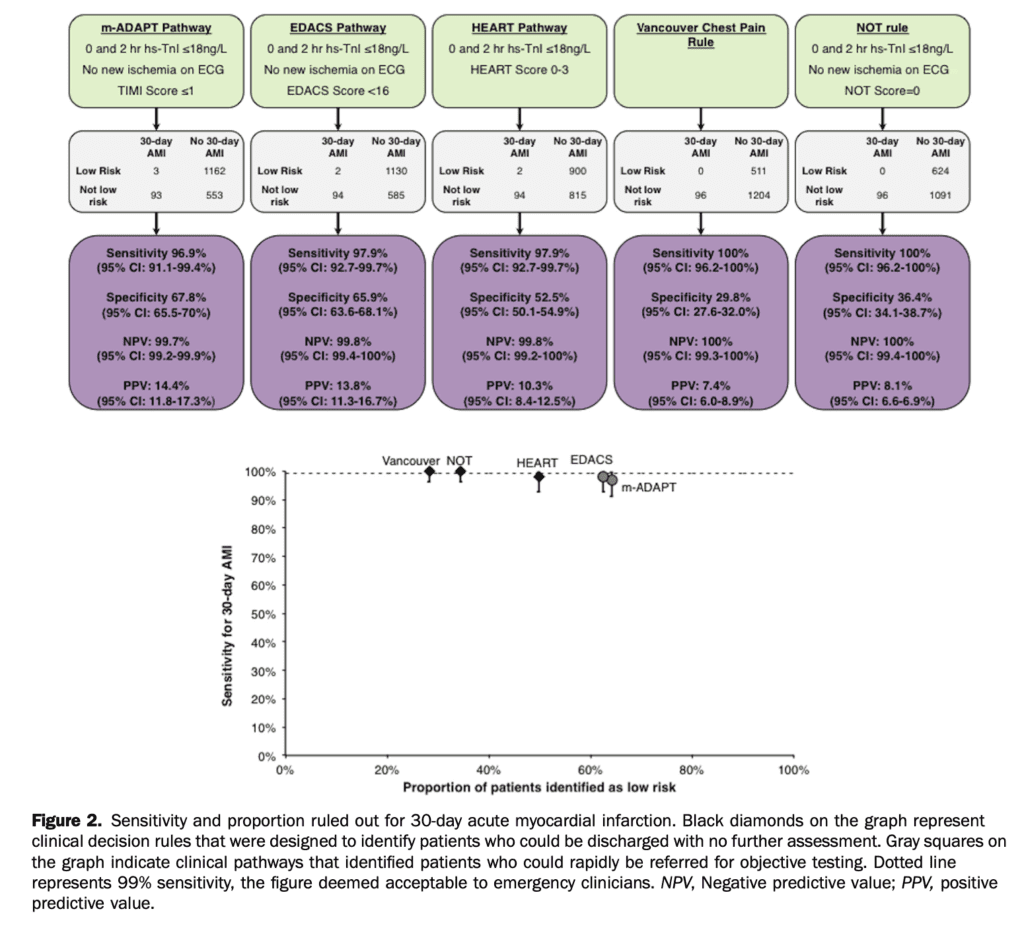
Sensitivity/specificity results for the clinical pathways for 30-day AMI:
- m-ADAPT: 3 false negatives, sensitivity 96.9% (95% CI 91.1% – 99.4%), specificity 67.8%
- EDACS: 2 false negatives, sensitivity 97.9% (95% CI 92.7% – 99.7%), specificity 65.9%
- HEART: 2 false negatives, sensitivity 97.9% (95% CI 92.7% – 99.7%), specificity 52.5%
- Vancouver Chest Pain Rule: 0 false negatives, sensitivity 100% (95% CI 96.2% – 100%), specificity 29.8%
- No Objective Testing Rule: 0 false negatives, sensitivity 100% (95% CI 96.2% – 100%), specificity 36.4%
-
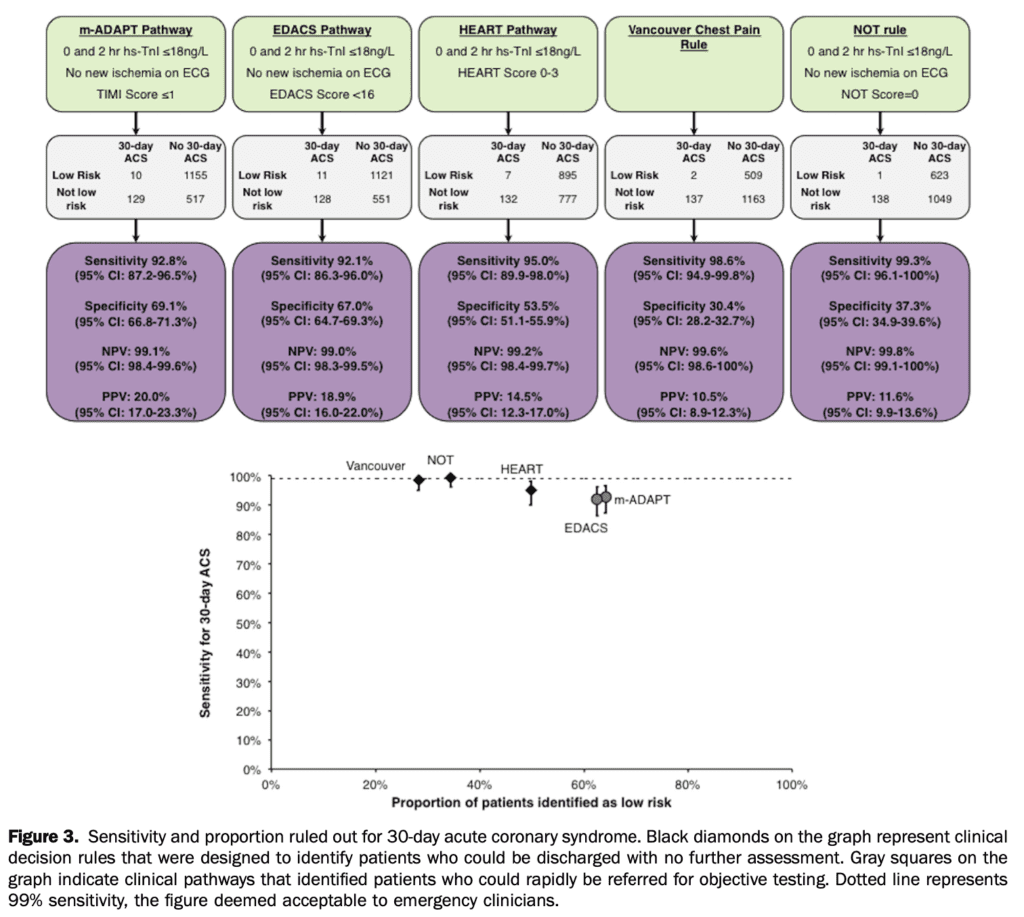
Sensitivity results for the clinical pathways for 30-day ACS:
- m-ADAPT: 10 false negatives, sensitivity 92.8% (95% CI 87.2% – 96.5%), specificity 69.1%
- EDACS: 11 false negatives, sensitivity 92.1% (95% CI 86.3% – 96.0%), specificity 67.0%
- HEART: 7 false negatives, sensitivity 95.0% (95% CI 89.9% – 98.0%), specificity 53.5%
- Vancouver Chest Pain Rule: 2 false negatives sensitivity 98.6% (95% CI 94.9% – 99.8%), 30.4%
- No Objective Testing Rule: 1 false negatives sensitivity (95% CI 96.1% – 100%), specificity 37.3% 99.3%
-
Proportion of patients with low-risk classification:
- m-ADAPT: 64.3%
- EDACS: 62.5%
- HEART pathway: 49.8%
- Vancouver Chest Pain Rule: 28.2%
- No Objective Testing rule: 34.5%.
Strengths:
- ED patient population in two large tertiary care centers
- Cardiologists were blinded to whether or not patients were low risk or not
- Cardiologists were blinded to Access hs-TnI data when assigning endpoints
- Cardiologists conducted a blind review of all patients who received a cardiac endpoint and 10% of cases with no cardiac endpoint
- No loss to follow up
- Thorough interrogation of follow-up medical record
Limitations:
- Data from two separate single-center studies in Australia whose patient population may be different than an individual ED population
- Decision rules were retrospectively calculated with high sensitivity troponin from datasets of two clinical decision rule derivation studies
- Validation was performed on the same patients
- Excluded undomiciled patients who represent a high-risk population
-
HEART pathway was not calculated as originally outlined
- May have changed the proportion of patients at low risk
- Diagnostic accuracy of m-ADAPT, EDACT, and NOT rule pathways may have been overstated as some of the patients in this study overlapped with those from which the rules were derived.
-
Selection bias present given enrollment was from 8 am to 5 pm.
- The authors argue that patients who present from outside of these hours do not significantly differ from those that do.
-
The incidence of AMI and ACS in this study was low,
- Which could affect the generalizability of the results.
- Beckman-Coulter provided assays and funding to cover the cost of research staff
- Authors received grants from Roche and Abbott diagnostics, and are paid, consultants
- Undomiciled patients were excluded and this group may be particularly high risk and vulnerable to follow-up loss.
Discussion:
-
CDI’s are key components of routine ED practice, especially regarding chest pain.
- We simply can not admit and perform stress tests or PCI on all patients with chest pain nor is it prudent to do so.
- However, when a new troponin assay is developed CDI’s require re-validation with the new assay.
-
In this study, the scores were evaluated against endpoints that matched their stated purpose.
- The investigators retrospectively applied the hs-TnI assay to 2 prior derivation studies. Derivation studies will often report validation on the same population.
- However, this represents a relatively low level of evidence for clinical prediction rules. Stronger evidence would require large multicenter prospective trials. (Important to note this article is from 2017)
- The patient population was from two EDs at tertiary care centers in Australia. Again, validation rules should be tested prospectively in large trials in a variety of clinical practice settings to ensure findings are not due to chance alone.
-
30-day MACE is a common endpoint in cardiology papers, but it is not without flaws
-
MACE is a composite of endpoints
- Death
- MI
- Stroke
- Hospitalization because of HF
- Revascularization via PCI or CABG
- These endpoints are not equivalent
- Additionally, the decision to perform a PCI is subjective and cardiologists dependent
-
MACE is a composite of endpoints
Author Conclusion: “In this cohort with a low prevalence of acute myocardial infarction and acute coronary syndrome, the use of Access hs-TnI results with either the Vancouver Chest Pain rule or the No Objective Testing Rule enabled approximately one-third of patients to be safely discharged after 2-hour risk stratification with no further testing. The use of this assay within the EDACS, m-ADAPT, or HEART rule pathways enabled more than half of ED patients to be rapidly referred for objective testing.”
Clinical Bottom Line:
The results from retrospective application of the Beckman-Coulter Access high sensitivity troponin assay in the Vancouver Chest Pain rule, NOT, m-ADAPT, EDACS, and HEART pathways were promising. Many of these decision rules have subsequently undergone further validation in prospective trials. In 2017 when this paper was released we would have suggested larger prospective trials be performed for further external validation before the widespread use of these CDI’s with hs-TnI.
Guest Post By:

Olayode Owoade, MD
PGY-2, Emergency Medicine Resident
Saint Joseph’s University Medical Center, Paterson New Jersey
Email: olayodeo@gmail.com

Marco Propersi, DO FAAEM
Assisstan Professor, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson New Jersey
Twitter: @marco_propersi
Joe Bove, DO
Attending Physician, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson New Jersey
Email: jjbove08@gmail.com
Steven Hochman, MD FACEP
Associate Professor, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson New Jersey
Twitter: @hochmast
References:
- National Hospital Ambulatory Medical Care Survey: 2017 emergency department summary tables. National Center for Health Statistics, 2020. [Link is HERE]
- Than M, Cullen L, Aldous S, et al. 2-Hour accelerated diagnostic protocol to assess patients with chest pain symptoms using contemporary troponins as the only biomarker: the ADAPT trial. J Am Coll Cardiol. 2012;59(23):2091-2098. [Link is HERE]
- Cullen L, Greenslade JH, Hawkins T, et al. Improved Assessment of Chest pain Trial (IMPACT): assessing patients with possible acute coronary syndromes. Med J Aust. 2017;207(5):195-200. [Link is HERE]
- Guyatt G, Rennie D, Meade M, Cook D. Users’ Guides To The Medical Literature. 3rd ed. McGraw-Hill Education; 2015. [Link is HERE]
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Retrospective Validation of High-Sensitivity Troponin with 5 Clinical Decision Instruments appeared first on REBEL EM - Emergency Medicine Blog.


