
 Background: Alteplase is a tissue plasminogen activator that is approved for use prior to thrombectomy in ischemic strokes with the goal of reperfusion to ischemic areas of the brain. Tenecteplase is a recombinant enzyme derived from alteplase that is more specific to fibrin and more resistant to inactivation by alteplase inhibitors. Tenecteplase is less expensive, can be administered at a faster rate than alteplase and has a longer half-life allowing for bolus dosing. Prior studies have shown similar to better outcomes with use of tenecteplase versus alteplase in patients with ischemic stroke.
Background: Alteplase is a tissue plasminogen activator that is approved for use prior to thrombectomy in ischemic strokes with the goal of reperfusion to ischemic areas of the brain. Tenecteplase is a recombinant enzyme derived from alteplase that is more specific to fibrin and more resistant to inactivation by alteplase inhibitors. Tenecteplase is less expensive, can be administered at a faster rate than alteplase and has a longer half-life allowing for bolus dosing. Prior studies have shown similar to better outcomes with use of tenecteplase versus alteplase in patients with ischemic stroke.
Campbell BCV et al. Tenecteplase versus Alteplase before Thrombectomy for Ischemic Stroke. NEJM; 378(17): 1573-1582. PMID: 29694815
Clinical Question: In patients with ischemic stroke who undergo thrombectomy is tenecteplase non-inferior to alteplase in establishing reperfusion?
Population:
- Adult patients
- Thrombolysis administered within 4.5 hours of onset of ischemic stoke
- Cerebral vascular occlusion on CT of internal carotid artery, first or second segment of middle cerebral artery or basilar artery
- Thrombectomy within 6 hours of stroke onset
- CT-perfusion mismatch for anterior circulation strokes (removed after 80 patients enrolled)
Intervention: tenecteplase (0.25mg/kg; max dose 25mg) prior to thrombectomy
Control: alteplase (0.9mg/kg; max dose 90mg) prior to thrombectomy
Outcomes:
-
Primary outcome:
-
Restoration of blood flow to greater than 50% of the involved territory or no retrievable thrombus during thrombectomy
- Used modified Treatment in Cerebral Ischemia classification (0 = no flow, 3 = normal flow)
- If no thrombectomy, perfusion of at least 50% of involved territory on CT perfusion 1-2 hours after thrombolysis
-
Restoration of blood flow to greater than 50% of the involved territory or no retrievable thrombus during thrombectomy
-
Secondary outcome:
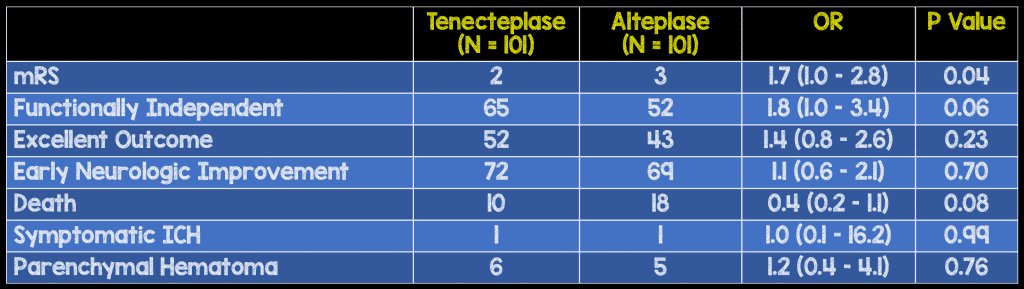
- Modified Rankin Scale (mRS) at 90 days
- Early neurologic improvement (reduction of NIHSS by at least 8 or a score of 0-1 at 72 hours)
- Death – any cause
- Symptomatic intracerebral hemorrhage
- Parenchymal hematoma
Design: Investigator-initiated, multi-center, prospective, randomized, open-label, blinded-outcome non-inferiority trial
Excluded: Severe preexisting disability (modified Rankin score of greater than 3)
Primary Results:
-
204 patients enrolled at 12 Australian and 1 New Zealand center between March 2015-October 2016
- 101 assigned to tenecteplase group
- 101 assigned to alteplase group
- 2 excluded (1 withdrew consent, 1 eligibility error)
- 6 patients had primary outcome measured by CT-perfusion only
-
Both groups had similar baseline characteristics
- Age (~70yo)
- Sex (~50% male)
- Middle cerebral artery occlusion most common (74 patients)
- Time to hospital presentation after symptom onset (~65 min)
- Time to thrombolytics (~130 min)
- Time to thrombectomy after thrombolytics (~55 min)
- Hospital transfer post-thrombolytics (~25% of patients)
Critical Results:
-
Substantial (> 50% restoration of blood flow) reperfusion (primary outcome)
- 22 in tenecteplase (22%) vs 10 in alteplase group (10%)
- Absolute difference: 12% (CI 2 – 21, p < 0.002)
- Incidence ratio: 2.2 (CI 1.1 – 4.4, p < 0.03)
- Odds ratio: 2.6 (CI 1.1 – 5.9, p < 0.02)
- NNT = 8
Secondary Results:
Strengths:
- Asks an important question that could potentially lead to a medication shift
- Included patients requiring transfer (increasing applicability)
- Adequate randomization
- Multicenter study
- Follow up was excellent
- Outcome assessors were blinded to treatment allocation
Limitations:
- Only included patients undergoing thrombectomy (13% of ischemic stroke patients)
- Median time from stroke to hospital arrival was longer in the alteplase group 72min vs 60min. This difference is of unclear importance
- Small sample size – only 204 patients enrolled over 19 months across 13 hospitals
- Trial was unblinded to clinicians
- Lack of transparency regarding patient enrollment (no flow chart)
- High mortality compared to symptom improvement
Author’s Conclusions: “Tenecteplase before thrombectomy was associated with a higher incidence of reperfusion and better functional outcome than alteplase among patients with ischemic stroke treated within 4.5 hours after symptom onset.”
Our Conclusions: Tenecteplase results in improved perfusion after thrombectomy compared to alteplase but only in patients that undergo endovascular intervention. Additionally, the rates of reperfusion prior to thrombectomy were low in both groups (22% in telecteplase vs 10% in alteplase group). There are no differences in clinically significant outcomes.
Potential Impact to Current Practice: This study only applies to a small subset of patients with strokes (ischemic, large-vessel, undergoing thrombectomy) so there is unlikely to be a difference in large population outcomes. However, hospital stroke outcomes are of increasing importance so if tenecteplase costs less and requires less time to administer, this data may lead to a shift in management.
Bottom Line: Neither alteplase or tenecteplase are very effective in significant vessel reperfusion. Though alteplase is the drug of choice currently in ischemic stroke, tenecteplase may provide more cost-effective and time-efficient option.
Guest Post By:

For More on This Topic Checkout:
- EM Lit of Note: The Futility of Alteplase
- The Bottom Line: EXTEND IA TNK
References:
- Parsons M, Spratt N, Bivard A, et al. A randomized trial of tenecteplase versus alteplase for acute ischemic stroke. N Engl J Med 2012; 366: 1099-107.
- Campbell BC, Mitchell PJ, Churilow L, et al. Tenecteplase versys alteplase before endovascular thrombectomy (EXTEND-IA TNK): A multicenter, randomized, controlled study. Int J Stroke 2018;13:328-34.
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Tenecteplase versus Alteplase before Endovascular Therapy for Ischemic Stroke (EXTEND-IA TNK) appeared first on REBEL EM - Emergency Medicine Blog.

