
 Background: Severely ill patients diagnosed with COVID-19 have an increased risk of cardiovascular complications, especially thromboembolic events (Bikdeli 2020). The overall incidence of developing venous thromboembolism (VTE) is approximately 17% in patients diagnosed with COVID-19, with a significantly higher rate in the ICU setting (Jiménez 2021).
Background: Severely ill patients diagnosed with COVID-19 have an increased risk of cardiovascular complications, especially thromboembolic events (Bikdeli 2020). The overall incidence of developing venous thromboembolism (VTE) is approximately 17% in patients diagnosed with COVID-19, with a significantly higher rate in the ICU setting (Jiménez 2021).
Multiple studies have investigated the use of antithrombotic agents in patients with COVID-19 admitted to various hospital settings (Talasaz 2021). Some of these papers have been reviewed on REBEL EM [Link is here] and [Link is here]. However, there is currently no evidence to support the use of antithrombotics in stable patients who are treated in the outpatient setting.
Some clinicians have extrapolated inpatient data and are using antithrombotics in the outpatient setting without evidence. How should we manage those symptomatic but stable patients with COVID-19 that are discharged home without an inpatient stay? Investigators of the ACTIV-4b trial sought to answer this question.
Article: Connors, J. M. et al. Effect of antithrombotic therapy on clinical outcomes in outpatients with clinically stable symptomatic COVID-19. JAMA 2021. PMID: 34633405
Clinical Question: Among symptomatic but clinically stable outpatients with COVID-19, does adding antithrombotic therapy, compared with placebo, reduce major cardiopulmonary adverse outcomes over a 45-day treatment period?
What They Did:
- Adaptive, randomized, double-blind, placebo-controlled trial
- 52 centers were included throughout the US for patient enrollment
- Study was conducted between September 2020 and June 2021, with final follow up as late as August 2021
- After randomization, patients received their study drug 24 to 72 hours later by mail
- Surveys and telephone calls were conducted to follow up with patients during the 45-day treatment period and 30 days post-treatment
Population:
-
Inclusion Criteria (Must have all of the following):
- Ambulatory patients ages 40 to 80 years old
- Recently diagnosed with COVID-19 via PCR or antigen test
- CrCl > 30 mL/min/1.73 m2
- Platelet count > 10,000/mm3.
- Exclusion Criteria:
-
- Patients previously hospitalized for COVID-19
- History of acute leukemia
- Recent major bleeding event
- Contraindications for anticoagulation
- Other indication for anticoagulation
- Other indication for single or dual antiplatelet therapy
- Pregnancy or lactating
- Inability to be contacted by telephone or other electronic methods to communicate
Intervention:
-
Patients randomized 1:1:1:1
- Treatment with aspirin (81 mg daily) with matching placebo (placebo to create twice daily dosing)
- Prophylactic dose of apixaban (2.5 mg twice daily)
- Therapeutic dose of apixaban (5 mg twice daily)
- Placebo (twice daily)
Control:
- Placebo taken twice daily
Outcomes:
-
Primary Outcome: Composite endpoint of:
- Symptomatic DVT
- Pulmonary embolism
- Arterial thromboembolism
- Myocardial infarction
- Ischemic stroke
- Hospitalization for a cardiovascular or pulmonary event
- All-cause mortality within 45 days
-
Secondary Outcome:
- Any individual component of the above primary outcome
- Mortality without a preceding hospitalization.
-
Principal Safety Outcomes:
- Major bleeding
- Clinically relevant non major bleeding (CRNMB) as defined by International Society on Thrombosis and Hemostasis (ISTH) criteria
- Disseminated intravascular coagulation (DIC)
Results:
- 775 symptomatic patients were initially identified with COVID-19 that were treated as outpatients
- 657 patients were randomized and included in the study
- Study was terminated early due to low event rates
- Only 558 patients had started treatment when the study was terminated
- Only patients that had initiated treatment were included in the result analysis
-
22 patients (3.3%) who were randomized became clinically unstable before initiating treatment and required hospitalization
- Two of these patients died from COVID-related respiratory failure during the 45 day observation period.
- One patient died from respiratory failure after the 45 day observation period, but during the 30 day safety follow-up period.
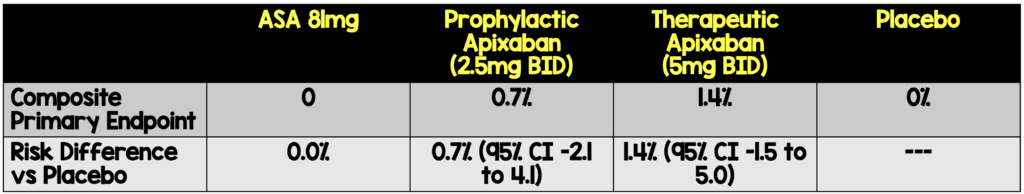
- The risk differences of the primary composite endpoint were not significant.
Absolute Risk Reductions of Treatment vs Placebo
Strengths
- Asks a patient-centered question, looking at reducing major cardiopulmonary adverse events within the first 45 days after treatment.
- The study population was diverse including 52 centers throughout the US.
- There was blind adjudication of the outcomes, allowing for limited bias when interpreting the results.
- Investigators performed post hoc analysis evaluating any medical event regardless of etiology during the 45 day follow-up period.
- The study was randomized, double-blinded with a placebo control minimizing the risk of bias
- Baseline characteristics were balanced across all groups.
- 99.6% of patients had complete follow-up at 45 days after drug initiation or at the time of trial termination.
Limitations
- Patients included were limited to ages 40 to 80 years old which severely limits the generalizability of the study, especially since younger patients are more likely to be stable and discharged home.
- Non-consecutive enrollment. It’s unclear how many patients were eligible for enrollment versus how many were contacted for enrollment.
- While inflammatory and thrombotic markers, such as D-dimer and CRP, were measured, these markers were not considered when determining who should receive therapy.
- There was a significant amount of time (up to 10 days or more) from diagnosis to initiation of the treatment, which could have affected the data and outcomes.
- The study was terminated after only 9% of the expected number of patients were enrolled.
- Primary Outcome was a composite where all pieces of the composite are not of equal clinical significance
- Truncated RCTs are at risk of overestimating results due to chance.
Discussion
- Overall, this study suggests that there is no benefit to antiplatelet or anticoagulant in the stable symptomatic COVID patient in the outpatient setting.
-
The primary outcome was a composite of 7 different outcomes.
- These outcomes ranged from asymptomatic DVT to mortality, which clearly have very different implications and importance for the patient.
- A composite outcome, by aggregating a number of widely different endpoints, can mask the significance of the more important patient-centered endpoints (death, severe disability, etc.).
-
The trial was terminated due to a much lower event rate than initially predicted.
- Investigators estimated an event rate between 4% and 8% but the actual event rate was less than 1%.
- Based on the initial calculations, 7000 patients were needed to reach a power of 80-90%.
- 1750 patients included in each of the four treatment groups
- However, only 558 patients in total across all four treatment groups were included in the analysis (approximately 165 in each treatment group).
- Therefore, the study is markedly underpowered to draw any conclusions.
-
Trials can be truncated for a few reasons including for benefit, apparent harm or for inability to demonstrate a treatment effect.
- In this case, the RCT was truncated due to a low event rate, or an inability to reveal treatment effect.
- With less than 9% of the expected number of enrolled patients to power the study, it is possible that the results are overestimated or underestimated.
-
D-dimer and CRP were measured but not accounted for when deciding treatment.
- The HEP-COVID Trial enrolled patients with D-dimer cutoff of 4x the upper limit of normal (among other criteria).
- How might the incorporation of inflammatory markers in patient selection affect the outcome?
-
3.3% of patients were hospitalized before beginning treatment.
- In this study, patients were deemed stable for discharge by the provider, however, no specific criteria were used to objectively determine who should be discharged.
- There was up to a 10-day period before treatment was initiated. It’s possible that this particular group of patients could have benefited from treatment sooner, potentially preventing hospitalization.
- While there were no major bleeding events, and only a small number of clinically relevant bleeding events, placing a patient on anticoagulation comes with risks and should be considered carefully.
Author’s Conclusions: “Among symptomatic clinically stable outpatients with COVID-19, treatment with aspirin or apixaban compared with placebo did not reduce the rate of a composite clinical outcome. However, the study was terminated after enrollment of 9% of participants because of a primary event rate lower than anticipated.”
Our Conclusions: The preliminary evidence in this study demonstrates no benefit from aspirin or apixaban for thromboprophylaxis in symptomatic patients who are clinically stable for outpatient management. However, the lack of adequate powering prevents us from drawing firm conclusions.
Clinical Bottom Line:
Antithrombotics should not routinely be prescribed to patients with COVID-19 that are clinically stable for outpatient management.
References:
- Bikdeli B, Madhavan MV, Jimenez D, et al. COVID-19 and Thrombotic or Thromboembolic Disease: Implications for Prevention, Antithrombotic Therapy, and Follow-Up: JACC State-of-the-Art Review. J Am Coll Cardiol. 2020;75(23):2950-2973. PMID: 32311448
- Jiménez, David et al. “Incidence of VTE and Bleeding Among Hospitalized Patients With Coronavirus Disease 2019: A Systematic Review and Meta-analysis.” Chest vol. 159,3 (2021): 1182-1196. PMID: 33217420
- Talasaz AH, Sadeghipour P, Kakavand H, et al. Recent Randomized Trials of Antithrombotic Therapy for Patients With COVID-19: JACC State-of-the-Art Review. J Am Coll Cardiol. 2021;77(15):1903-1921. PMID: 33741176
For More on This Topic Checkout:
- Critical Care Reviews: mpRCT Anticoagulation Trial
- St. Emlyn’s Blog: Thromboprophylaxis for the Non ICU Hospitalised COVID-19 Patient
Guest Post By:

Jessica DiPeri, MD
PGY-2, Emergency Medicine Resident
Saint Joseph’s University Medical Center, Paterson New Jersey
Email: jtilzer12@gmail.com

Marco Propersi, DO FAAEM
Assistant Professor, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson New Jersey
Twitter: @marco_propersi
Steven Hochman, MD FACEP
Associate Professor, Emergency Medicine
Saint Joseph’s University Medical Center, Paterson New Jersey
Twitter: @hochmast
Post-Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The ACTIV-4b Trial: Antithrombotics For Treatment of Outpatient COVID-19 appeared first on REBEL EM - Emergency Medicine Blog.

