
 Background: Patients presenting with acute exacerbations of congestive heart failure are frequently treated with intravenous loop diuretics. Despite being treated with loop diuretics, the problem is many are discharged from the hospital with residual clinical signs of volume overload despite optimal treatment. One option to improve diuresis may be the addition of acetazolamide, however evidence supporting this strategy is sparse.
Background: Patients presenting with acute exacerbations of congestive heart failure are frequently treated with intravenous loop diuretics. Despite being treated with loop diuretics, the problem is many are discharged from the hospital with residual clinical signs of volume overload despite optimal treatment. One option to improve diuresis may be the addition of acetazolamide, however evidence supporting this strategy is sparse.
Paper: Mullens W et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. NEJM 2022. PMID: 36027559 [Access on Read By QxMD]
Clinical Question: Does the addition of acetazolamide to loop diuretics lead to more and faster decongestion in patients with acute decompensated heart failure with volume overload?
What They Did:
- Acetazolamide in Decompensated Heart Failure with Volume Overload (ADVOR) trial
- Multicenter, parallel-group, double-blind, randomized, placebo-controlled trial
- Performed at 27 sites in Belgium
- Patients with acute decompensated heart failure, clinical signs of volume overload (i.e. edema, pleural effusion, or ascites), and an N-terminal pro-B-type natriuretic peptide level of >1000pg/mL or a B-type natriuretic peptide level of >250pg/mL randomized to:
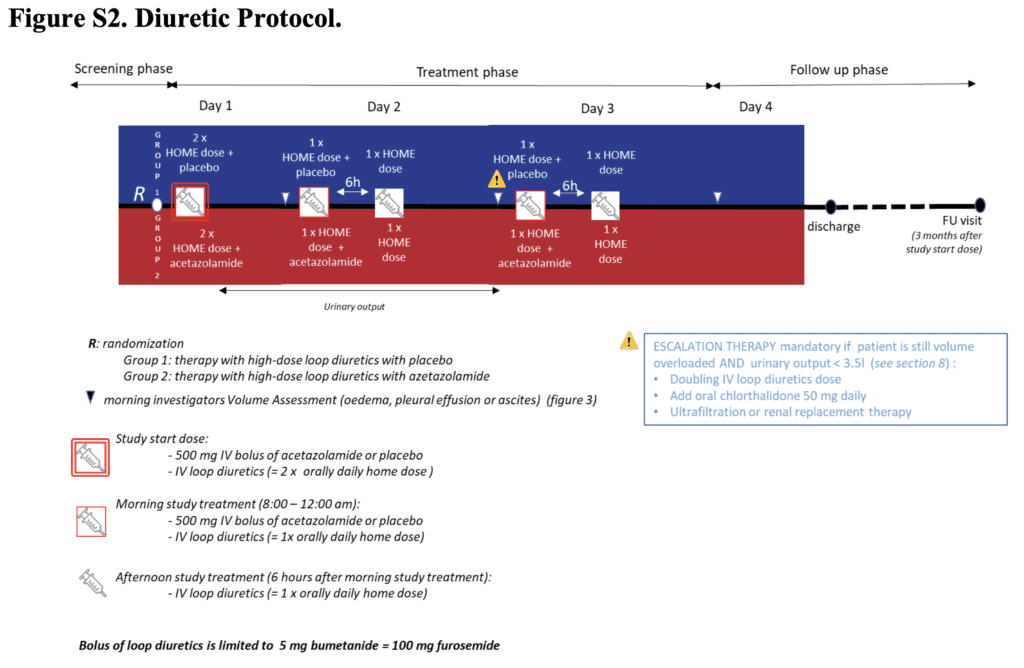
- IV acetazolamide (500mg qD)
- IV placebo
- Either was added to IV loop diuretics (at a dose equivalent to twice the oral maintenance dose)
- Given x3d or until occurrence of complete decongestion
- Randomization stratified to left ventricular ejection fraction (≤40% or >40%)
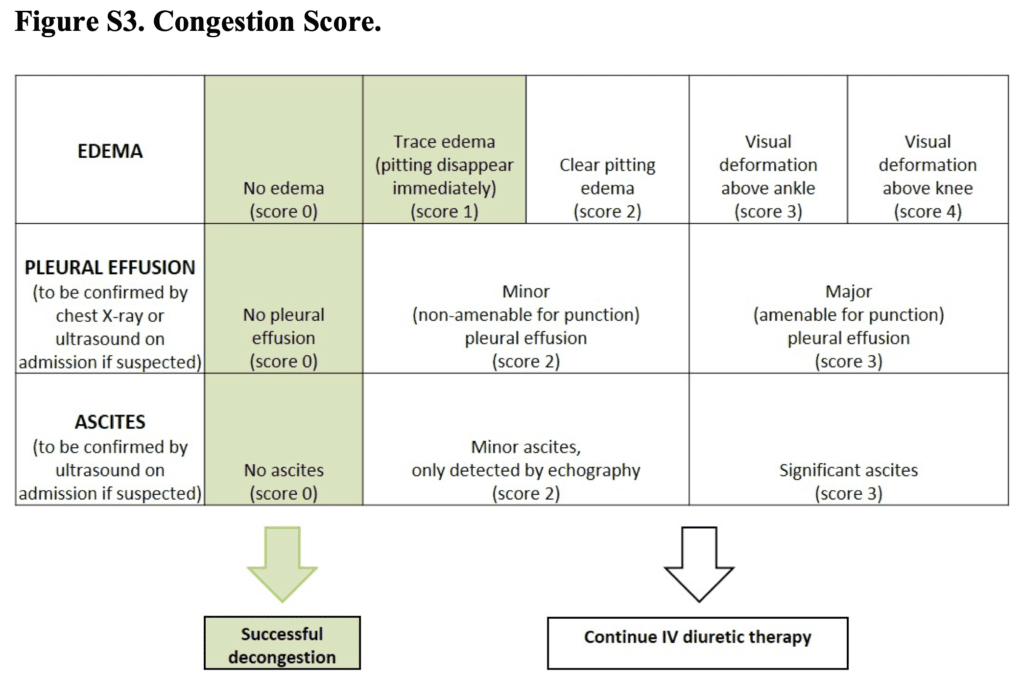
- Congestion Score (Range from 0 to 10):
- Sum of scores for the degree of edema
- Edema 0 to 4
- Pleural effusion 0 to 3
- Ascites 0 to 3
- Higher scores indicating worse condition
Outcomes:
-
Primary: Successful decongestion
- Defined as the absence of signs of volume overload, within 3d after randomization and without an indication for escalation of decongestive therapy
-
Key Secondary:
- Composite of death from any cause or rehospitalization for heart failure during 3 months of follow up
Inclusion:
- Adult patients
- Admitted to the Hospital due to acute decompensated heart failure
- At least one clinical sign of volume overload (i.e. edema, pleural effusion, or ascites)
- NT-proBNP >1000pg/mL or BNP >250pg/mL
- Patients also required to be on at least 40mg of furosemide or an equivalent dose (1mg bumetanide or 20mg of torasemide) for at least 1 month before randomization
Exclusion:
- Receipt of acetazolamide maintenance therapy or treatment with another proximal tubular diuretic including a sodium-glucose cotransporter 2 (SGLT2) inhibitor
- SBP <90mmHg
- eGFR <20mL/min/1.73m2
- Treatment with an IV loop diuretic at a dose of more than 80mg of furosemide equivalent during the index hospitalization was not allowed before randomization
Results:
- 519 patients randomized
- Median NT-proBNP: 6173 pg/mL (Range 3068 to 10m896)
- Median congestion score of 4
- Edema of the lower extremities was the most prevalent sign of volume overload
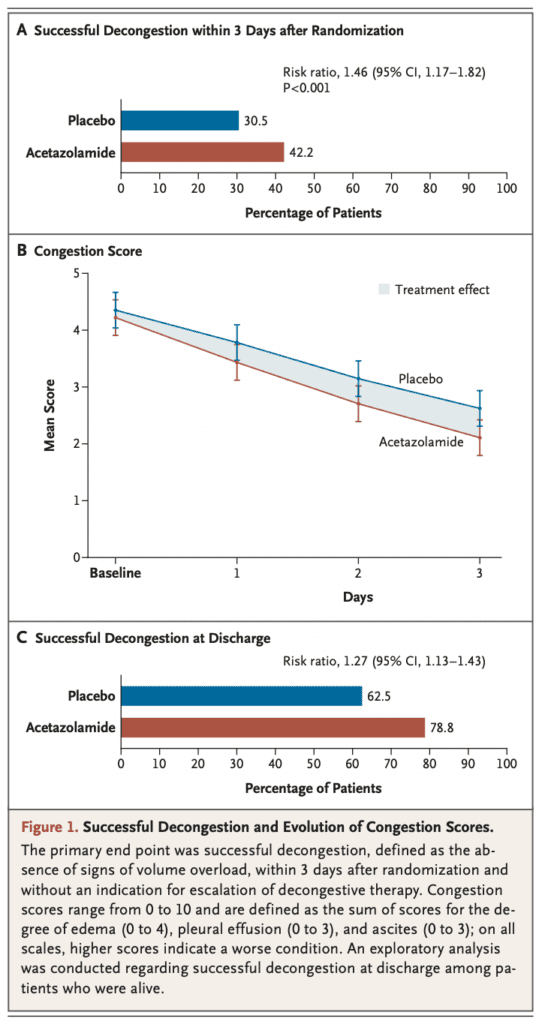
- Successful Decongestion (Primary Outcome):
- Acetazolamide: 42.2%
- Placebo: 30.5%
- RR 1.46; 95% CI 1.17 to 1.82; p <0.001
- Death From Any Cause OR Rehospitalization for Heart Failure:
- Acetazolamide: 29.7%
- Placebo: 27.8%
- HR 1.07; 95% CI 0.78 to 1.48
- Duration of hospital stay 9.9d in the placebo group compared to 8.8d in the acetazolamide group
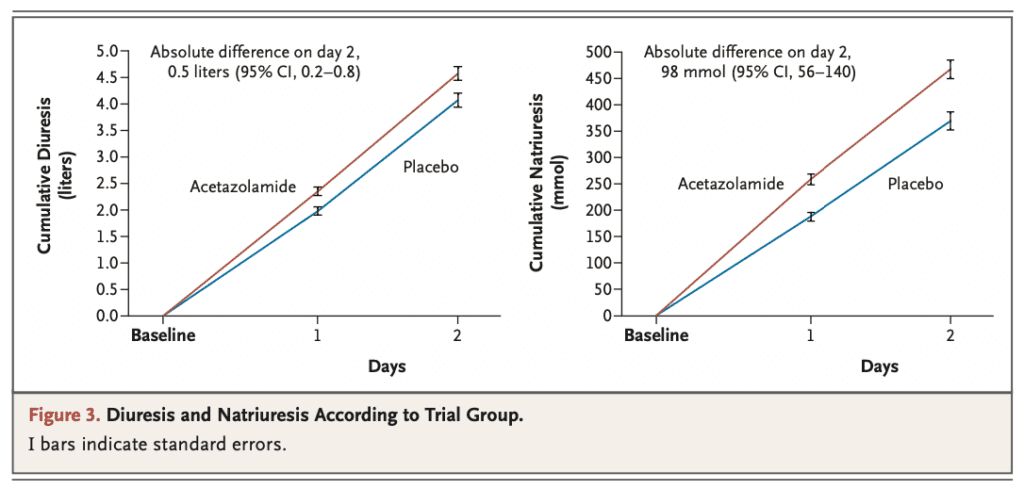
- Acetazolamide treatment was associated with higher cumulative urine output consistent with better diuretic efficiency
- Incidence of worsening kidney function, hypokalemia, hypotension, and adverse events was similar between groups
Strengths:
- Asks a clinically meaningful question
- Multicenter, parallel-group, double-blinded, randomized, placebo-controlled trial
- Groups balanced at baseline in terms of vital signs, congestion, NYHA functional class, renal function, coexisting conditions, and other treatments
Limitations:
- Predominantly Caucasian group of patients which could limit generalizability to other racial/ethnic groups
- Patients enrolled were already on treatment for CHF, and therefore this strategy may not be generalizable to new onset heart failure patients
- Patients with GFRs <20mL/min/1.73m2 were excluded from the trial making the utility of acetazolamide in this group of patients unclear
- A single furosemide regimen was used throughout the study (i.e. BID dosing).As seen in the subgroup analysis, a higher dose or more frequent dosing of furosemide could lead to less effectiveness of acetazolamide
Discussion:
- The addition of acetazolamide to standard IV loop diuretic therapy was associated with a higher incidence of successful decongestion within 3-days after randomization
- Additionally, patients treated acetazolamide had more diuresis and natriuresis, shorter hospital stay, and more likely to be discharged without residual signs of volume overload compared to placebo
- The benefit of acetazolamide treatment in terms of decongestion was maintained at discharge in a higher percentage of patients being discharged from the hospital without residual congestion (Difference of 16.3% compared to placebo)
- Residual congestion is linked to adverse outcomes and rehospitalization, therefore an aggressive strategy that helps with diuresis is an important one
- The rate of death or rehospitalization was lower than prior studies:
- ADVOR Trial [1]: ≈28% at 90d
- DOSE Trial [2]: 50% at 60d
- CARRESS-HF Trial [3]: 40% at 60d
- Natriuresis vs Diuresis
- Natriuresis = Excretion of sodium in the urine
- Diuresis = Excretion of urine
- Furosemide alone can cause more retention of sodium resulting in more dilute urine (i.e. diuresis NOT natriuresis)
- A common clinical practice in the ICU is to use a loop diuretic as monotherapy or a loop diuretic in combination with a thiazide diuretic.This can commonly lead to hypernatremia, hypochloremia, and a metabolic alkalosis. Therefore, many clinicians often stop diuretic therapy early because of these side effects
- Some will start adding free water either intravenously or through an NG/OG tube to decrease sodium levels thus defeating the purpose of diuresis
- Others will eventually switch to acetazolamide once the loop diuretics causes a profound metabolic alkalosis. Acetazolamide will cause a metabolic acidosis and therefore reverse the effects of the metabolic alkalosis caused by loop diuretics. It seems logical to use a combination of acetazolamide and a loop diuretic early in the course of diuresis to avoid these unwanted side effects caused by loop diuretics and/or thiazides
Author Conclusion: “The addition of acetazolamide to loop diuretic therapy in patients with acute decompensated heart failure resulted in a greater incidence of successful decongestion.”
Clinical Take Home Point: In patients with acute decompensated heart failure, and clinical signs of volume overload (i.e. edema, pleural effusion, or ascites) the addition of 500mg of IV acetazolamide to standard loop diuretic therapy resulted in more diuresis, more natriuresis, shorter hospital stay, and an increased likelihood of being discharged without residual signs of volume overload.
References:
- Mullens W et al. Acetazolamide in Acute Decompensated Heart Failure with Volume Overload. NEJM 2022. PMID: 36027559 [Access on Read By QxMD]
- Felker GM et al. Diuretic Strategies in Patients with Acute Decompensated Heart Failure. NEJM 2011. PMID: 21366472
- Bart BA et al. Ultrafiltration in Decompensated Heart Failure with Cardiorenal Syndrome. NEJM 2021. PMID: 23131078
For More Thoughts on This Topic Checkout:
- PulmCrit: PulmCrit Hot Take – Acetazolamide Plus Furosemide for Decongestion of Heart Failure (ADVOR Trial)
Post Peer Reviewed By: Frank Lodeserto, MD
The post The ADVOR Trial: Acetazolamide in Acute Decompensated Heart Failure appeared first on REBEL EM - Emergency Medicine Blog.
