
 Background: Respiratory support is a common intervention in pediatric ICUs and can include HFNC and CPAP/BPAP to avoid invasive mechanical ventilation. HFNC has become more popular due to its ease of use, perceived patient comfort, and the ability to discharge patients still receiving HFNC out of the ICU. Despite its growing popularity there is limited randomized clinical trial evidence to support the use of HFNC as a 1st line modality in acutely ill children.
Background: Respiratory support is a common intervention in pediatric ICUs and can include HFNC and CPAP/BPAP to avoid invasive mechanical ventilation. HFNC has become more popular due to its ease of use, perceived patient comfort, and the ability to discharge patients still receiving HFNC out of the ICU. Despite its growing popularity there is limited randomized clinical trial evidence to support the use of HFNC as a 1st line modality in acutely ill children.
Paper: Ramnarayan P et al. Effect of High-Flow Nasal Cannula Therapy vs Continuous Positive Airway Pressure Therapy on Liberation From Respiratory Support in Acutely Ill Children Admitted to Pediatric Critical Care Units: A Randomized Clinical Trial. JAMA 2022. PMID: 35707984 [Access on Read by QxMD]
Clinical Question: In acutely ill children requiring non-invasive ventilation (NIV) in a pediatric ICU, is 1st line use of HFNC noninferior to CPAP in terms of time to liberation from all forms of respiratory support?
What They Did:
- First-Line Support for Assistance in Breathing in Children (FIRST-ABC)
- 2 protocols in one
- Step-Up [1]: Results reported in this post
- Step-Down [2]: HFNC did not meet criterion for noninferiority (Not reported in this post)
- Evaluate the noninferiority of HFNC as 1st line mode of NIV for acute illness, compared to CPAP for time to liberation from all forms of respiratory support in children
- Pragmatic, unblinded, parallel-group, multicenter, randomized noninferiority clinical trial
- 24 pediatric ICUs in the UK
- Patients randomized to:
- HFNC
- CPAP 7 to 8cmH20
- Noninferiority margin of an adjusted HR of 0.75 (Corresponds to a median 16hr increase in time to liberation for HFNC)
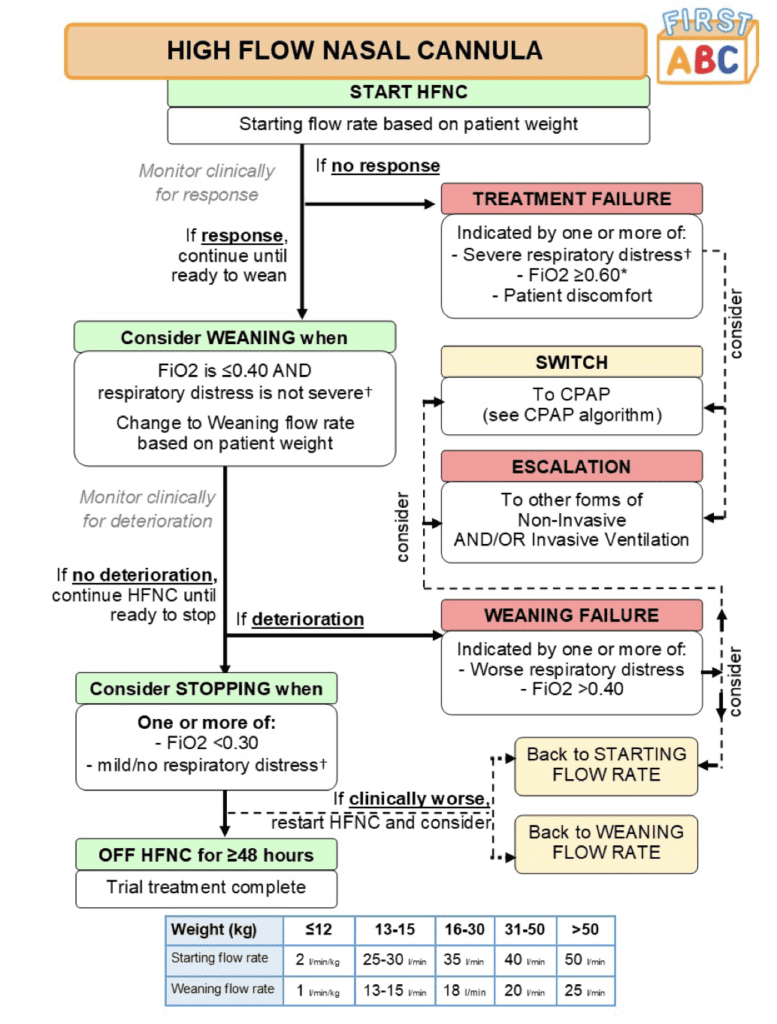
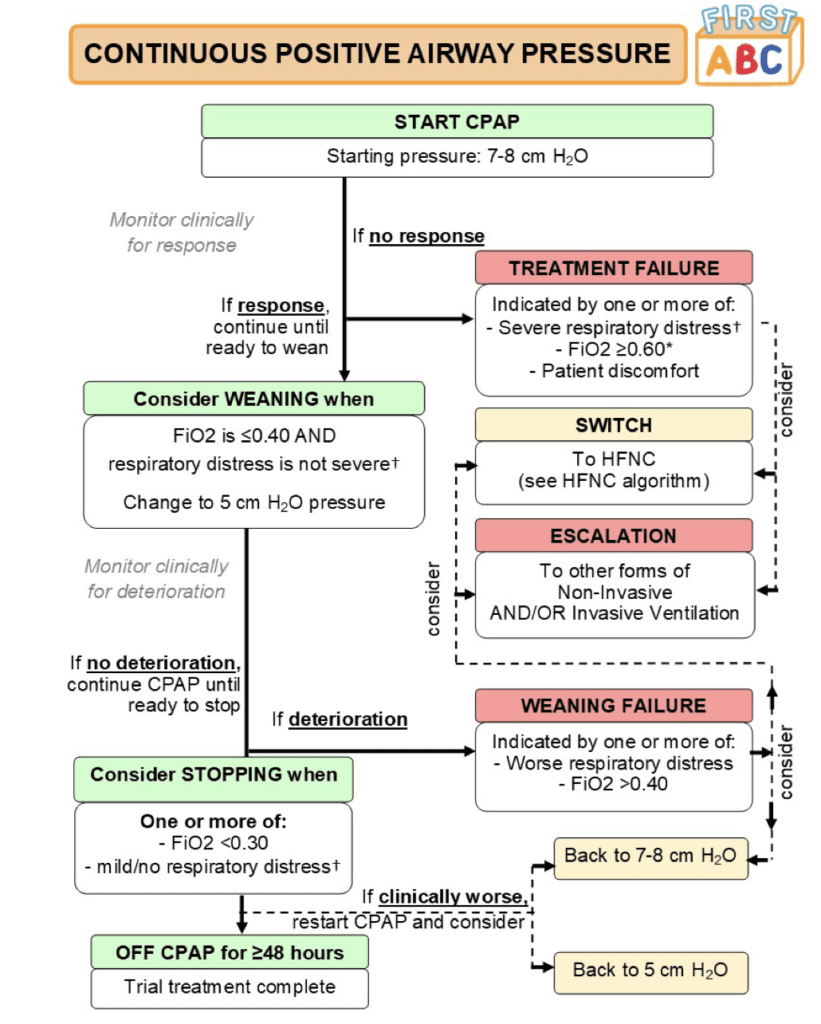
Outcomes:
- Primary: Time from randomization to liberation from respiratory support (Defined as the start of a 48hr period during which a participant was free from all forms of respiratory support (invasive or noninvasive) but excluding supplemental oxygen)
-
Key Secondary (There Were 7 Total Secondary Outcomes):
- Mortality at ICU discharge
- Intubation within 48hrs
- Use of sedation during noninvasive respiratory support
Inclusion:
- Age birth (>36wks corrected gestational age) up to 15 years
- Admitted or being admitted to critical care unit
- Treating clinician felt noninvasive respiratory support necessary for an acute illness
Exclusion:
- Clinical decision to start a mode other than CPAP or HFNC (i.e. noninvasive ventilation)
- Receipt of either CPAP or HFNC for more than 2hrrs prior to randomization
- Preadmission receipt of domiciliary respiratory support
Results:
- 600 children randomized
- 573 patients included in primary analysis
- Median age: 9 months
- Main reason for admission = Bronchiolitis (≈50%)
- Treatment failure requiring either a switch or escalation:
- HFNC: 33.1% after median 6.1hrs following randomization
- CPAP: 53.3% after median 4.5hrs following randomization
- More patients switched from CPAP to HFNC (30.9%) than from HFNC to CPAP (20.0%)
- Reasons for switching were mainly related to clinical deterioration in the HFNC group and patient discomfort in the CPAP groups
- Children you switched from CPAP to HFNC for discomfort reasons were older compared with children who did not switch (Median age: 12mos vs 3mos)
-
Median Time to Liberation from Respiratory Support:
- HFNC: 52.9hrs (95% CI 46.0 to 60.9hrs)
- CPAP: 47.9hrs (95% CI 40.5 to 55.7hrs)
- Absolute Difference: 5hrs; 95% CI -10.1 to 17.4hrs
- Adjusted HR 1.03; 1-sided 97.5% CI 0.86 to infinity
- This met the criterion for non-inferiority
- Use of Sedation:
- HFNC: 27.7%
- CPAP: 37.0%
- Adjusted OR 0.59; 95% CI 0.39 to 0.88
- Mean Duration of ICU Stay:
- HFNC: 5d
- CPAP: 7.4d
- Adjusted Mean Difference -3d; 95% CI -5.1 to -1d
- Mean Duration of Hospital Stay
- HFNC: 13.8d
- CPAP: 19.5d
- Adjusted Mean Difference -7.6d; 95% CI 0-13.2 to 01.9d
- Intubation within 48hrs:
- HFNC: 15.4%
- CPAP: 15.9%
- Adjusted OR 0.99; 95% CI 0.61 to 1.62
- Mortality at ICU Discharge:
- HFNC: 1.7%
- CPAP: 1.5%
- Adjusted HR 1.03; 95% CI 0.86 to 1.22
- Most Common Adverse Event (Nasal Trauma):
- HFNC: 2.0%
- CPAP: 6.5%
Strengths:
- Asks a clinically important question about interventions frequently performed in pediatric patients in respiratory distress
- Largest RCT comparing 2 commonly used modes of NIV respiratory support in children
- Study conducted in a heterogenous group of acutely ill children increasing generalizability
- Bias minimized by specifying the same weaning criteria for HFNC and CPAP, same number of weaning steps, minimum twice daily clinical evaluations to assess progression, and online training to strengthen protocol adherence
- Groups well balanced in terms of age, sex, reason for admission, noninvasive strategy at admission, level of respiratory distress, and vital signs
- In both groups the allocated treatment was started in the majority of patients who started respiratory support (HFNC 98.3% and CPAP 88.5%)
- Performed several sensitivity analyses to test robustness of primary outcome
- Majority of patients were under 1 year of age (many times pediatric studies primarily enroll older kids); this can also be seen as a weakness in terms of applicability to older children (particularly teens/adolescents)
Limitations:
- Clinicians not blinded to treatment which could bias decisions to switch, escalate treatment, or start respiratory support at all
- ≈1000 patients were excluded from analysis due to >2hrs of prior noninvasive respiratory support
- Non-consecutive enrollment: ≈850 patients were eligible but not enrolled
- Multicenter study, but not multinational
- Majority of patients with bronchiolitis reducing generalizability to other pathologic processes
- Non-inferiority approach is weaker when a trial is unblinded, particularly since the decision to switch/escalate is subjective
Discussion:
- First line use of HFNC in acutely ill children admitted to the pediatric ICU and assessed to require noninvasive respiratory support was noninferior compared to CPAP for time from randomization to liberation from respiratory support
- Above finding is in contrast to the FIRST-ABC Step-Down RCT [2], in which HFNC was not found to be noninferior to CPAP in children extubated after invasive mechanical ventilation
- Possible explanations for this:
- There was a high rate of treatment failure with CPAP in this trial compared to the STEP-Down RCT (52.3% vs 33.7%)
- More children switched from CPAP to HFNC mostly due to patient discomfort (30.9% vs 12.3%) in this trial
- Proportion of children switched from HFNC to CPAP was similar in both trials (20.0% vs 23.5%)
- ≈25% of the CPAP groups were still receiving CPAP 24hrs after starting treatment vs ≈50% of the HFNC group were still receiving HFNC in the same timeframe
- Although hypothesis generating, subgroup analysis showed that the effect of HFNC differed between children already on vs not already on respiratory support at randomization (i.e. The point estimate was consistent with inferiority of HFNC for those already receiving support). This needs to be evaluated further in future trials
Author Conclusion: “Among acutely ill children clinically assessed to require noninvasive respiratory support in a pediatric critical care unit, HFNC compared with CPAP met the criterion for noninferiority for time to liberation from respiratory support.”
Clinical Take Home Point: Based on these results, in acutely ill children admitted to the pediatric ICU assessed to require noninvasive respiratory support, 1st line HFNC, or CPAP are both reasonable for time from randomization to liberation from respiratory support. One thing to consider in making this decision is, HFNC resulted in less use of sedation for comfort, ICU and hospital length of stay compared to CPAP.
References:
- Ramnarayan P et al. Effect of High-Flow Nasal Cannula Therapy vs Continuous Positive Airway Pressure Therapy on Liberation From Respiratory Support in Acutely Ill Children Admitted to Pediatric Critical Care Units: A Randomized Clinical Trial. JAMA 2022. PMID: 35707984 [Access on Read by QxMD]
- Ramnarayan P et al. FIRST-ABC Step-Down RCT Investigators and Paediatriic Critical Care Society Study Group. Effect of High-Flow Nasal Cannula Therapy vs Continuous Positive Airway Pressure Following Extubation on Liberation from Respiratory Support in Critically Ill Children: A Randomized Clinical Trial. JAMA 2022. PMID: 35390113
For More Thoughts on This Topic Checkout:
- Don’t Forget the Bubbles: FIRST-ABC Step Down – High Flow After Extubation
- Don’t Forget the Bubbles: FIRST ABC Step Up – High Flow in Acutely Unwell Children
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post The FIRST-ABC Step Up Trial: HFNC vs CPAP for Liberation of Respiratory Support in Children? appeared first on REBEL EM - Emergency Medicine Blog.
