
 Background: Patients with COVID-19 are at increased risk from thromboembolic phenomena. Patient-specific factors such as comorbidities and immobility have been linked to thrombosis. Disease-specific factors such as virus-induced endothelial changes and cytokine storm may also be triggers. Furthermore, patients with elevated inflammatory markers such as D-dimer levels are at an increased risk from thrombosis.
Background: Patients with COVID-19 are at increased risk from thromboembolic phenomena. Patient-specific factors such as comorbidities and immobility have been linked to thrombosis. Disease-specific factors such as virus-induced endothelial changes and cytokine storm may also be triggers. Furthermore, patients with elevated inflammatory markers such as D-dimer levels are at an increased risk from thrombosis.
Early studies on the utility of therapeutic-dosing of anticoagulation vs prophylactic-dosing were inconsistent but a recent multiplatform clinical trial demonstrated a benefit in hospitalized patients that did not require critical care. Conversely, the same multiplatform study did not show a benefit for critical care patients. [Link is here]. REBEL EM recently reviewed two of these papers [Link is here]. The HEP-COVID randomized clinical trial adds to the growing body of evidence supporting therapeutic-anticoagulation for preventing thrombosis in noncritically ill patients hospitalized with COVID-19.
Paper: Spyropoulos AC et al. Efficacy and Safety of Therapeutic-Dose Heparin vs Standard Prophylactic or Intermediate-Dose Heparins for Thromboprophylaxis in High-risk Hospitalized Patients With COVID-19: The HEP-COVID Randomized Clinical Trial [published online ahead of print, 2021 Oct 7]. JAMA Intern Med 2021. [PMID: 34617959]
Clinical Question: Does thromboprophylaxis with therapeutic-dose low-molecular-weight heparin reduce the incidence of major thromboembolism and death compared with prophylactic/ intermediate-dose heparins in inpatients with high-risk COVID-19?
What They Did
- Multicenter, pseudo-open label, active control randomized clinical trial
- Screened patients for inclusion within 72 hours
- Randomization was performed using a secure web application
- Patients were stratified based on noncritical care (nonintensive care unit [ICU]) or critical care (ICU) status at the time of randomization
-
ICU status was defined by:
- Mechanical ventilation
- Noninvasive positive pressure ventilation or high-flow nasal cannula
- Vasopressors
- Vital sign monitoring more often than every 4 hours
- Participants were randomly assigned 1:1 to therapeutic-dose enoxaparin or institutional standard prophylactic/ intermediate-dose heparins
- Patients and investigators were blinded to treatment assignment as much as possible
- Study drug was administered for the duration of hospitalization, including patient transfers to ICU settings
- All patients underwent laboratory and screening lower extremity compression ultrasonography testing at hospital day 10 + 4
Intervention
- Patients in the therapeutic-dose group received enoxaparin at a dose of 1 mg/kg subcutaneously twice daily if CrCl was ≥30 mL/min/1.73 m2 or 0.5 mg/kg twice daily if CrCl was 15-29 mL/min/1.73 m2
- If CrCl fell below 15 mL/min/ 1.73 m2, enoxaparin was converted to treatment-dose intravenous UFH until kidney function improved to CrCl greater than 15 mL/min/1.73 m2, when blinded-dose subcutaneous enoxaparin was resumed
Control
-
Patients in the standard-dose group received prophylactic or intermediate-dose heparin regimens per local institutional standard and could include:
- UFH, up to 22,500 IU subcutaneously (divided twice or three times daily)
- Enoxaparin 30 mg or 40 mg subcutaneously once or twice daily (weight-based enoxaparin 0.5 mg/kg subcutaneously twice daily was permitted but strongly discouraged)
- Dalteparin, 2500 IU or 5000 IU subcutaneously daily
Outcomes
Primary Outcome: Composite of:
-
Venous Thromboembolism
- Symptomatic upper or lower extremity deep vein thrombosis
- Asymptomatic lower-extremity proximal deep vein thrombosis
- Symptomatic pulmonary embolism
- Splanchnic vein thrombosis
- Cerebral sinus thrombosis
-
Arterial Thromboembolism
- Myocardial infarction
- Ischemic stroke
- Peripheral or systemic ATE
- Death from any cause within 30 ± 2 days after randomization.
Secondary:
- Composite primary outcome within 14 days after admission
- Progression to acute respiratory distress syndrome
- New-onset atrial fibrillation
- Acute kidney injury
- Nonfatal cardiac arrest
- Endotracheal intubation
- Extracorporeal membrane oxygenation
- Rehospitalization within 30 ± 2 days.
Primary Major Safety Outcome:
- Major bleeding based on the International Society on Thrombosis and Haemostasis criteria within 30 ± 2 days after randomization
Inclusion
- Hospitalized nonpregnant adults 18 years or older with COVID-19 diagnosed by nasal swab or serologic testing
- Requirement for supplemental oxygen per investigator judgment
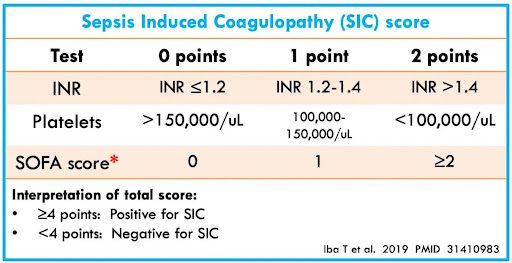
- Plasma D-dimer level greater than 4 times the upper limit of normal based on local laboratory criteria or a sepsis-induced coagulopathy score of 4 or greater [Link is here]
Exclusion
- Physician determined need for full-dose anticoagulation or dual antiplatelet therapy
- Bleeding within the past month
- Active gastrointestinal or intracranial cancer
- Bronchiectasis or pulmonary cavitation
- Hepatic dysfunction with baseline international normalized ratio (INR) > 1.5
- Creatinine clearance (CrCl) < 15 mL/min/1.73 m2
- Platelet count less than 25 000/μL
- History of heparin-induced thrombocytopenia (HIT) within 100 days
- Hypersensitivity/intolerance to study drugs or components
Results
-
11649 patients assessed for eligibility
- 10618 did not meet eligibility criteria
- 548 declined to participate
- 257 randomized
- 253 included in the analysis
- In the per-protocol population, therapeutic-dose LMWH reduced the incidence of the primary efficacy outcome (48.0% vs 30.1%; RR, 0.63; 95% CI, 0.44–0.89; P = .007), with a reduction in thromboembolism (33.3% vs 10.6%; RR, 0.32; 95% CI, 0.18–0.58; P < .001) vs standard-dose heparins
- In the overall population, the number needed to treat to prevent 1 thromboembolic event and death was 8, while in the non-ICU stratum, the number needed to treat was 5.
- The number needed to harm in the overall population was 33, while in the non-ICU stratum, the number needed to harm was approximately 2000
Primary Outcome:
-
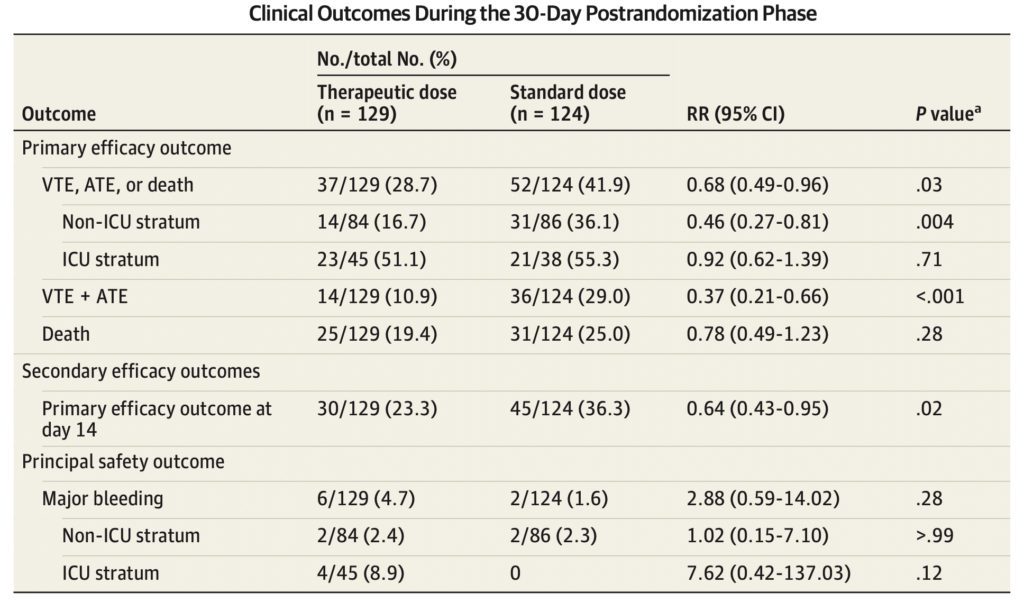
In the modified intention-to-treat population, 89 patients (35.2%) reached a primary efficacy outcome
- 56 deaths (22.1%)
- 50 thromboembolic events (19.8%)
-
The incidence of the primary efficacy outcome was 41.9% in the standard-dose group vs 28.7% in the therapeutic-dose group (RR, 0.68; 95% CI, 0.49–0.96; P = .03) (Statistically Significant)
- Driven by a reduction in thromboembolism (29.0% vs 10.9%; RR, 0.37; 95% CI, 0.21–0.66; P < .001) (Statistically Significant)
- Majority of thromboembolic events consisted of symptomatic deep vein thrombosis and nonfatal pulmonary embolism
- No significant difference in death between groups (25.0% vs 19.4%; RR, 0.78; 95% CI, 0.49–1.23; P = .28)
- More cardiovascular deaths in the standard-dose group vs therapeutic-dose group (12.1% vs 7.8%; RR, 0.64; 95% CI, 0.30–1.37; P = .25)
-
8 major bleeding events (3.2%), 2 (1.6%) in the standard-dose vs 6 (4.7%) in the therapeutic-dose groups (RR, 2.88; 95% CI, 0.59–14.02; P = .17
- No major bleed events were fatal
- Therapeutic-dose LMWH reduced the incidence of the primary efficacy outcome among patients in the non-ICU stratum compared to standard-dose heparins (36.1% vs 16.7%; RR, 0.46; 95% CI, 0.27–0.81; P = .004) (Statistically Significant)
- Therapeutic-dose LMWH did not reduce the incidence of the primary efficacy outcome among patients in the ICU stratum compared to standard-dose heparins (55.3% vs 51.1%; RR, 0.92; 95% CI, 0.62–1.39; P = .71).
- There was no significant difference in major bleeding between groups in either stratum
- More major bleeds among patients in the ICU stratum in the therapeutic-dose compared with the standard-dose group (4 [8.9%] vs 0; RR, 7.62; 95% CI, 0.42–137.03; P = .12)
Secondary Outcome:
- For secondary outcomes, therapeutic-dose LMWH reduced the incidence of the primary efficacy outcome at day 14 from hospitalization (36.3% vs 23.3%; RR, 0.64; 95% CI, 0.43–0.95; P = .02) (Statistically Significant)
- There were no significant differences in other secondary outcomes between groups
- In each of the 2 groups, there were 3 patients who had more than 1 thromboembolic event
- Serious adverse events included 1 case of thrombocytopenia with negative HIT serology results and 1 case of rectus sheath hematoma, both in the therapeutic-dose group
Primary Major Safety Outcome:
- There was no statistically significant difference in major bleeding in the therapeutic-dose group (4.7%) vs the standard-dose group (1.6%) (RR 2.88; CI 0.59–14.02; P = .28)
Strengths
- Multicenter study (12 centers total)
- Randomized patients consecutively
- Diverse patient population
- Outcomes were patient-oriented
- Performed intention to treat and per-protocol analysis
- Actively screened VTE in all patients which can eliminate some bias that may have occurred by unblinding
- No patients were lost to follow up
Limitations
- While the investigators sought to blind clinicians, it’s unclear if blinding was successful based on the manuscript and it’s likely some unblinding occurred potentially biasing results.
- The study pharmacists as well as data abstractors and designated randomization personnel (i.e., research coordinators and/or research nurses performing the randomization process) were unblinded.
-
The therapeutic dose was compared to prophylactic and intermediate dosing which may dilute the effect of therapeutic dosing.
- It’s unclear why a patient in the control arm was given prophylactic or intermediate and this introduces bias into the study.
- Lots of patients were screened but only a very small number met inclusion criteria.
-
The investigators changed the study design twice
- D-dimer cutoff from 6 x upper limit of normal to 4 x upper limit of normal
- hypoxemia criterion changed from respiratory rate greater than 20 breaths/min, and oxygen saturation less than 92% on room air to any perceived need for supplemental oxygen as per investigator judgment.
- Specific subset population may affect generalizability and utility
-
The primary outcome was a composite of VTE, ATE, and Death
- Not all outcomes are equal
- Investigators assumed a relative risk reduction of 40% but they only achieved 32% rendering the study underpowered
Prior Data
-
The positive findings in this trial are congruent with a recent publication of multiplatform trials that showed a benefit of therapeutic anticoagulation in reducing disease severity.
- Therapeutic anticoagulation with heparin in noncritically ill patients with COVID-19. [PMID: 34351721]
-
However, the findings are contrary to other recent studies that showed no improvement in clinical outcomes with therapeutic anticoagulation.
- The INSPIRATION Randomized Clinical Trial [PMID: 33734299]
- The ACTION Randomized Clinical Trial [PMID: 34097856]
- Therapeutic anticoagulation with heparin in critically ill patients with COVID-19 [PMID: 34351722]
- Heparin for Moderately Ill Patients with Covid-19 [PMID: 34268513]
Discussion
-
The primary outcome is a composite outcome and not all components are equivalent.
- There are a number of important long-term chronic sequelae which may be caused by DVT and PE.
- Patients likely won’t care if they have a DVT or PE if they are dead.
-
The relative risk (RR) for the composite primary outcome (VTE, ATE, and death at 30 days) was 0.67.
- The risk of developing thromboembolism and death with therapeutic dose anticoagulation was two-thirds the risk of standard-dose anticoagulation.
- Statistically significant reduction was driven almost entirely by a reduction in thromboembolism in the therapeutic-dose anticoagulation cohort.
- The majority of thromboembolic events consisted of symptomatic deep vein thrombosis and nonfatal symptomatic pulmonary embolism.
- Not entirely surprising since the investigators were actively screening for VTE.
-
There was a trend toward mortality benefit in the therapeutic dose anticoagulation group.
- Risk difference of 5.6% was not statistically significant
- The study was not powered to find a mortality benefit
- Approx 1450 patients would be required to demonstrate a statistically significant mortality benefit
-
Additionally, no benefit was found in patients managed in an ICU setting.
- The authors postulate that heparins may only be beneficial early in the course of disease.
- Possibly before the advent of an irreversible hyperinflammatory state and cytokine storm causing thromboinflammation.
-
When evaluating only VTE and ATE the RR was 0.37.
- The risk of developing thromboembolism with therapeutic dose anticoagulation was just more than one-third of the risk of standard-dose anticoagulation.
- Therapeutic-dose anticoagulation reduced the relative risk (RRR) of thromboembolism by nearly two-thirds compared to standard-dose anticoagulation.
- The risk difference was 18%.
-
Investigators assumed a relative risk reduction of 40% but achieved 32%
- Sample size 80% larger, at 453 patients, would be required for adequate power to have confidence in the results
- Based on the study treating 5 non-ICU patients with therapeutic-dose anticoagulation may prevent one thromboembolic event.
- Moreover, we would need to treat 2000 patients to cause one episode of major bleeding.
-
It’s worth noting that the patient population who would benefit is a very specific subset potentially limiting use and generalizability.
- 93% of patients with COVID-19 screened did not meet inclusion criteria.
- Of the 253 patients who remained in the study, the benefit is seen only among the 170 patients who are sick enough to be hospitalized but not sick enough to be managed in the ICU.
Author’s Conclusion: “In the HEP-COVID randomized clinical trial, therapeutic-dose LMWH reduced the composite of thromboembolism and death compared with standard heparin thromboprophylaxis without increased major bleeding among hospitalized patients with COVID-19 with very elevated D-dimer levels. The treatment effect was not seen in ICU patients.”
Clinical Bottom Line:
This small trial showed promising evidence that therapeutic-dose LMWH reduced the composite outcome of death and thromboembolism when compared to the standard-dose in patients with markedly elevated D-dimer levels who were on oxygen therapy and not treated in an ICU. The benefit was not seen in patients treated in the ICU. The outcome was driven by the reduction in thromboembolism with no statistically significant difference in mortality. The study results are congruous with results from a recent multiplatform trial which also showed benefit with therapeutic-dose anticoagulation in reducing disease severity in noncritically ill patients.
References:
- Guyatt G, Rennie D, Meade M, Cook D. Users’ Guides To The Medical Literature. 3rd ed. McGraw-Hill Education; 2015. [Link is here]
- ATTACC Investigators; ACTIV-4a Investigators; REMAP-CAP Investigators, et al. Therapeutic Anticoagulation with Heparin in Noncritically Ill Patients with Covid-19. N Engl J Med. 2021;385(9):790-802. [PMID: 34351721]
- INSPIRATION Investigators, Sadeghipour P, Talasaz AH, et al. Effect of Intermediate-Dose vs Standard-Dose Prophylactic Anticoagulation on Thrombotic Events, Extracorporeal Membrane Oxygenation Treatment, or Mortality Among Patients With COVID-19 Admitted to the Intensive Care Unit: The INSPIRATION Randomized Clinical Trial. JAMA. 2021;325(16):1620-1630. [PMID: 33734299]
- Lopes RD, de Barros E Silva PGM, Furtado RHM, et al. Therapeutic versus prophylactic anticoagulation for patients admitted to hospital with COVID-19 and elevated D-dimer concentration (ACTION): an open-label, multicentre, randomised, controlled trial. Lancet. 2021;397(10291):2253-2263. [PMID: 34097856]
- REMAP-CAP Investigators; ACTIV-4a Investigators; ATTACC Investigators, et al. Therapeutic Anticoagulation with Heparin in Critically Ill Patients with Covid-19. N Engl J Med. 2021;385(9):777-789. [PMID: 34351722]
- Sholzberg M, Tang GH, Rahhal H, et al. Heparin for Moderately Ill Patients with Covid-19. Preprint. medRxiv. 2021;2021.07.08.21259351. Published 2021 Jul 12. [PMID: 34268513]
For More on This Topic Checkout:
- REBEL EM: COVID-19 and Anticoagulation: Full Dose or Prophylactic Dose?
- Critical Care Reviews: mpRCT Anticoagulation Trial
- St. Emlyn’s Blog: Thromboprophylaxis for the Non ICU Hospitalised COVID-19 Patient
Post-Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The HEP-COVID Trial: Therapeutic Anticoagulation in Non-Critically Ill COVID-19 Patients appeared first on REBEL EM - Emergency Medicine Blog.
