
 Background: The cornerstones of sepsis management continues to include early identification, early appropriate empiric antibiotics, definitive source control, and vasopressors to support end organ perfusion. There have been multiple studies looking at the co-administration of hydrocortisone, ascorbic acid, and thiamine (known as HAT therapy or the metabolic cocktail) to help reduce mortality and reverse shock. Despite the original Marik study showing an association between HAT therapy and a 31.9% overall decrease in mortality and a 3-fold decrease in time to vasopressor discontinuation in patients presenting with severe sepsis and septic shock, the mortality benefit has not been reproduced in subsequent randomized clinical trials. Studies focused specifically on the use of corticosteroids have demonstrated reduced time on vasopressors in patients in septic shock. The bigger question is does vitamin C and thiamine add anything additional to help improve mortality (The ORANGES Trial)?
Background: The cornerstones of sepsis management continues to include early identification, early appropriate empiric antibiotics, definitive source control, and vasopressors to support end organ perfusion. There have been multiple studies looking at the co-administration of hydrocortisone, ascorbic acid, and thiamine (known as HAT therapy or the metabolic cocktail) to help reduce mortality and reverse shock. Despite the original Marik study showing an association between HAT therapy and a 31.9% overall decrease in mortality and a 3-fold decrease in time to vasopressor discontinuation in patients presenting with severe sepsis and septic shock, the mortality benefit has not been reproduced in subsequent randomized clinical trials. Studies focused specifically on the use of corticosteroids have demonstrated reduced time on vasopressors in patients in septic shock. The bigger question is does vitamin C and thiamine add anything additional to help improve mortality (The ORANGES Trial)?
Paper: Iglesias J et al. Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis: The ORANGES Trial. Chest 2020. PMID: 32194058
Clinical Question: Does hydrocortisone, ascorbic acid, thiamine (HAT) therapy improve clinical outcomes in sepsis and septic shock?
What They Did:
- Randomized, double-blinded, placebo-controlled trial conducted from February 2018 to June 2019
- Performed in two community non-teaching hospitals in the US
- Patients were randomized to:
- HAT: Ascorbic Acid 1500 mg q6hr, thiamine 200 mg q12hr, and hydrocortisone 50 mg q6hr for a maximum of 4 days
- Placebo: mating saline placebo for a maximum of 4 days
- Intensivists were allowed to use open-label corticosteroid therapy for patients as deemed necessary for usual care (i.e. respiratory failure)
- Study medications were discontinued if patients were discharged from the ICU before 4 days
Outcomes:
-
Primary:
- Resolution of shock (Time from starting blinded study medications to discontinuation of all vasopressor support)
- Change in SOFA score (initial SOFA score minus the day 4 SOFA score)
-
Secondary:
- 28d mortality
- ICU mortality
- Hospital mortality
- Procalcitonin clearance (PCT-c) = initial PCT minus PCT at 96hrs divided by the initial PCT multiplied by 100
- Hospital LOS
- ICU LOS
- Ventilator-free days = uber of days free of mechanical ventilation up until day 28
- Acute kidney injury = Increase in serum creatinine (SCr) >0.3mg/dL, or a level >1.5x the baseline value or initiation of renal replacement therapy
-
Safety:
- Serum creatinine
- Urine oxalate = 24-hour urine collection on day 4
- Adverse reactions
Inclusion:
- Adults ≥18 years of age
- Primary diagnosis of sepsis or septic shock (according to the 2016 Surviving Sepsis Campaign definitions)
- Diagnosis of sepsis or septic shock within 12hrs of admission into the ICU
- Compliance with 3-hour sepsis bundle (There was an update in 2018 reducing the time of the bundle to 1 hour instead 3 hours, however the 3-hour time frame was maintained due to patient enrollment already being started)
Exclusion:
- Age <18 years
- Pregnancy
- Do not resuscitate or do not intubate order on admission
- Terminal end stage disease (i.e. stage IV cancer, end-stage heart failure)
- Did not have primary admitting diagnosis of sepsis or septic shock
- Required immediate surgery
- Had HIV and a CD4 <50mm2
- Known glucose-6 phosphate dehydrogenase deficiency
- Transferred from another hospital
- Presented with sepsis or septic shock >24 hours from admission
Results:
- 137 patients were randomized
- Most patients received 1st dose of study treatment between 3 and 14 hours (mean 9.9 +/- 4.5hrs) from presentation to the ED
- Major sources of infection were:
- Pulmonary: 43%
- Urogenital: 31%
- Primary bacteremia: 14%
- At time of enrollment 50% of patients were on mechanical ventilation and 75% were on vasopressors
- Mean SOFA score was 8.1 +/- 3.3 and APACHE II score was 24.5 +/- 8.2 which gives an estimated mortality of 34% +/- 2%
- 64% of patients had low or severe vitamin C deficiency at presentation
-
Resolution of Shock (Co-Primary Outcome):
- HAT: 27 +/- 22hrs
- Placebo: 53 +/- 38hrs
- P<0.001
- Adjusting for corticosteroid use, HAT therapy remained significant in resolution of shock (mean time to discontinuation of vasopressors and reversal of shock was 44hrs). The mean time in HAT therapy was 34 hours vs 54 hours in the placebo arm
-
Change in SOFA Score (Co-Primary Outcome):
- HAT: 3 (1 to 6)
- Placebo: 2 (0 to 4)
- P = 0.17
- No significant differences found in ICU/hospital mortality, ICU/hospital LO, ventilator free days, or PCT-c
- Renal outcomes were similar in both arms in terms of AKI with 79% in the HAT arm and 75% in the placebo arm (p = 0.68; OR 0.79; 95% CI 0.35 to 1.77)
- No adverse events were noted that were deemed related to the study drug
Strengths:
- Randomized, double-blinded, placebo-controlled trial
- No baseline differences between groups in terms of demographics, comorbidities, laboratory values APACHE II scores, or SOFA scores
- Used an intention-to-treat analysis which mirrors daily clinical practice
Limitations:
- Small cohort size not allowing for detection in differences of hospital mortality and length of stay
- Based the reduction of time to vasopressor discontinuation on the flawed Marik study (54hrs +/-30hrs vs 30hrs)
- 41% of patients in the placebo arm received corticosteroids which would affect the outcome of time to vasopressor discontinuation
- More patients in the HAT arm were on vasopressors (82%) compared to the placebo arm (68%) which could bias the results. It is unclear how this would bias the results, but baseline differences will have an effect on outcomes
Discussion:
- There was a difference in time to shock reversal in this study, however the ranges are broad and overlap. This makes this information less valuable at the bedside
- According to clinicaltrials.gov the study was submitted on January 30th, 2018 and on June 11th, 2019 the primary outcome was changed from in-hospital mortality to time to vasopressor independence (hours) and change in SOFA score. This change was made after all the data was collected. It is unclear if the authors had analyzed the data or not, however it is suspicious when a change is made this late in the process. Of note the original primary outcome was not statistically significant making this change even more suspect

- The above information suggests data dredging (collecting the data, looking for a difference in one of the many outcomes that were sought out and then pushing the one where there is a difference to the lead the story). If you have a lot of outcomes you investigate, you will find a difference in some of then simply by random chance alone. Thus, secondary endpoints should be hypothesis generating, which is what the primary outcomes should be
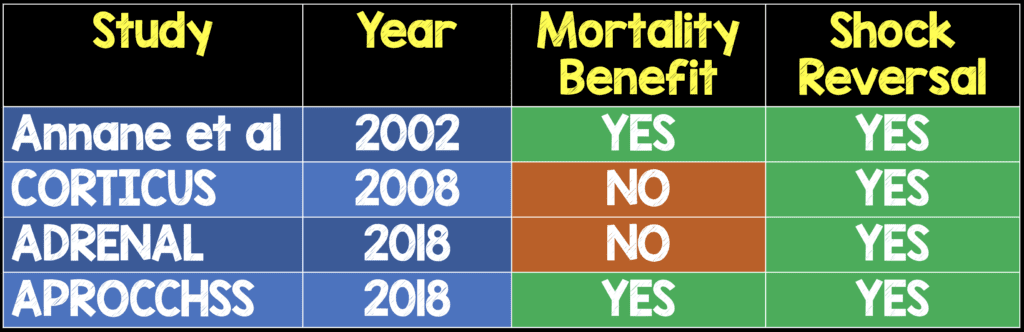
- It’s no surprise that we see a reversal of shock with HAT therapy compared to placebo. Hydrocortisone itself used in septic shock has been shown to improve shock (see table below). The authors adjusted for corticosteroid administration in the comparator group. The authors state that there was a synergistic effect of vitamin C in the reversal of shock and augmentation of hemodynamic effects.
- Interestingly, in this study there was no difference in SOFA score or PCT clearance, both of which are non-patient-oriented outcomes. The authors propose the reason for this is that there was less severity of vitamin C deficiency compared to previous studies:
- ORANGES: 21.7 +/- 14.8umol/L
- Marik study [2]: 14.7 +/-11.8umol/L
- Fowler et al [3]: 17.9 +/- 2.4 umol/L
- Although there was increased oxalate excretion in the HAT grop vs placebo group, there did not appear to be any differences between the two groups in the development of AKI
Author Conclusion: “Our results suggest that the combination of IV ascorbic acid, thiamine, and hydrocortisone significantly reduced the time to resolution of shock. Additional studies are needed to confirm these findings and assess any potential mortality benefit from this treatment.”
Clinical Take Home Point: In this small trial where the primary outcome was changed after full data collection (and likely analysis) was complete, HAT therapy appears to decrease vasopressor duration. However, the methodological flaws in the study relegate this information to hypothesis generating only and should not influence our clinical care at this time. Based on prior, better designed studies (i.e. VITAMINS, etc…), HAT therapy should not be part of routine care in sepsis.
References:
- Iglesias J et al. Outcomes of Metabolic Resuscitation Using Ascorbic Acid, Thiamine, and Glucocorticoids in the Early Treatment of Sepsis: The ORANGES Trial. Chest 2020. PMID: 32194058
- Marik PE et al. Hydrocortisone, Vitamin C, and Thiamine for the Treatment of Severe Sepsis and Septic Shock: A Retrospective Before-After Study. CHEST 2017. PMID: 27940189
- Fowler AA et al. Phase I Safety Trial of Intravenous Ascorbic Acid in Patients with Severe Sepsis. J Transl Med 2014. PMID: 24484547
For More Thoughts on This Topic Checkout:
- REBEL EM: The Marik Protocol – Have We Found a “Cure” for Severe Sepsis and Septic Shock?
- REBEL EM: CITRIS-ALI – Vitamin C in Patients with Sepsis and Severe Acute Respiratory Failure
- REBEL Cast: Ep74 – Is it all About the VITAMINS in Sepsis?
- REBEL EM: The HYVCTTSSS Trial – The “Metabolic Cocktail” in Another RCT
<a href=”https://www.medforums.com/blogs/rebel-em” title=”MedForums – Read One . Rate One . Review One”><img alt=”Leave us a review on MedForums” src=”https://static.medforums.com/images/partner-images/review-medforums-blue.svg” width=”300px”></a>
The post The ORANGES Trial: Why You Can’t Just Read the Abstract appeared first on REBEL EM - Emergency Medicine Blog.
