

Background: Primary spontaneous pneumothorax, by definition, occurs without trauma or any underlying lung pathology. Often patients are otherwise young and healthy. However, management continues to be debated and may lead to unnecessary hospitalization. Previous studies regarding ambulatory management included randomized trials that lacked adequate power, and recently, a 2013 systematic review that, although showed promise in the ambulatory approach, had poor data quality with a high risk of bias. (Brims 2013) The RAMPP trial is the first large-scale randomized controlled trial to delineate whether ambulatory management of primary spontaneous pneumothorax can help reduce hospitalization compared to standard care.
Paper: Hallifax RJ et al. Ambulatory management of primary spontaneous pneumothorax: an open-label, randomised controlled trial. Lancet. 2020;396(10243):39-49. PMID: 32622394
Clinical Question: Can ambulatory management of patients with primary spontaneous pneumothorax reduce the length of hospitalization?
What They Did:
- Open-label, multicenter, randomized controlled trial of patients with symptomatic primary spontaneous pneumothorax recruited from 24 UK hospitals over 3 years.
- Patients were randomly assigned 1:1 to treatment with either an ambulatory device or standard guideline-based management (aspiration, standard chest tube insertion, or both).
Population:
Inclusion criteria:
- 16-55 yo age
- Symptomatic spontaneous pneumothorax
-
Or a large pneumothorax
- ≥ 2cm interpleural distance at the level of the hilum
Exclusion criteria:
- Known or suspected underlying lung disease (Included > 20-pack-year tobacco use)
- Tension pneumothorax
- Contraindications to a thoracic procedure
- Pregnant or lactating
Intervention:
- Ambulatory device: 8F catheter attached to a self-contained, one-way Heimlich valve and fluid collection chamber.
- Inserted either in the second intercostal space midclavicular or the fifth intercostal space midaxillary.
- Patients were observed for 1-2 hours for clinical stability, and placement was confirmed with a chest x-ray.
-
Patients were discharged if:
- Stable cardiopulmonary observations
- No increase in the size of the pneumothorax since the last radiograph
- Did not require oxygen or other respiratory support
- Able to self-care
- Provided a point of contact and follow-up plan
- Lives with a responsible person at home and agrees with disposition.
-
Patients followed up every 1-2 days till day 4.
- The device was removed if there was sufficient reexpansion (<1cm of apical air on chest X-ray) and no air leak.
Control:
-
Standard treatment as per the 2010 BTS Pleural Guidelines.
- If pneumothorax size is >2cm and/or the patient appears breathless → Use 14-16G cannula to aspirate <2.5L → If the pneumothorax is <2cm and breathing improved → Discharge with outpatient pulmonary follow-up in 2-4 weeks.
- If aspiration is unsuccessful based on the criteria above → Chest tube is inserted (size 8-14Fr) and admitted to the hospital.
Outcomes:
Primary Outcome: Total length of hospital stay up to 30 days after randomization, including initial hospital stay and readmissions.
Secondary Outcomes:
- The need for further pleural procedures (any intervention that punctured the pleura after the first treatment intervention).
- Occurrence of adverse events
- Pain and breathlessness VAS scores
-
Recurrence rates
- New episode of symptomatic pneumothorax after full resolution of chest radiograph or occurring after 1-week follow-up visit.
- Time off work because of pneumothorax treatment.
Results:
- 776 patients were screened
- 236 were enrolled and randomized
- 117 to Ambulatory group
-
119 to Standard Care group
- 6 patients from the stand care group were excluded from the analysis because data was not available
- 12 lost to follow-up from each group
-
More patients in the standard care arm:
- Had previous pneumothorax (27% vs. 22%)
- Had previous procedures to treat a pneumothorax (89% vs. 82%)
- Had a family history of pneumothorax (11% vs. 6%)
- Were ex-tobacco smokers (22% vs. 15%)
- Were ex-marijuana smokers (24% vs. 17%)
-
More patients in the ambulatory care arm:
- Were current tobacco smokers (52% vs. 47%)
- Never smoked marijuana (49% vs. 46%)
- Were current smokers of marijuana (30% vs. 26%)
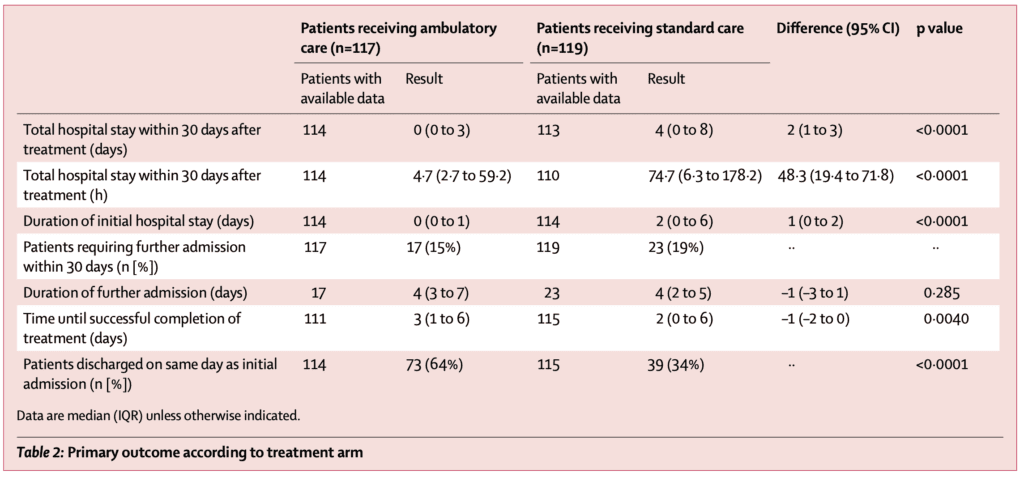
Primary Outcome:
- At day 30, the median hospitalization was 0 days [IQR 0–3] in 114 patients in the ambulatory care cohort vs. 4 days [IQR 0–8] in the 113 patients who received standard care; p<0.0001
Secondary Outcomes:
- 24 (21%) patients in the ambulatory care arm and 42(35%) patients in the standard care arm required additional procedures; p=0.0075
- 64 (55%) patients in the ambulatory care arm and 46 (39%) patients in the standard care arm had adverse events; p<0.0001
- All 14 serious adverse events occurred in patients who received ambulatory care
- Eight (57%) serious adverse events were related to the intervention, including an enlarging pneumothorax, asymptomatic pulmonary edema, and the device malfunctioning, leaking, or dislodging.
Strengths:
- Randomized controlled, multicenter study increases external validity.
- Investigators asked a patient-oriented research question.
- Randomization was done through a centralized, computer-generated algorithm.
- The study was well-powered, with target participants reaching 236 total patients.
- Patients in the study groups were similar concerning known prognostic factors and demographics.
- Exclusion and inclusion criteria helped filter patients at higher risk of further complications from pneumothorax.
- Patients were recruited primarily from the ED
- High level of compliance: Ambulatory group (97%) and standard care (95%)
- Performed a worst-case scenario analysis on patients with missing info and those excluded from the analysis.
- A validated scoring system was used to measure the secondary outcome of pain and shortness of breath.
- Prespecified criteria were established to determine if medical treatment failed.
- An equal number of patients were lost to follow-up between the two groups.
Limitations:
- Performed in a single country, limiting external validity as risk factors may vary between patient populations, and other countries may not have the same resource availability.
- By nature of the interventions, the study could not be blinded, which can lead to reporting and confirmation bias.
- The randomized cohort represents a convenience sample. Do patients with a pneumothorax who were not enrolled carry the same risk profile as those enrolled?
- Patients in the standard-of-care arm could still be discharged from the hospital if they were successfully treated with aspiration.
- Patients in the standard-of-care arm could be treated with aspiration or chest tube—two very different interventions.
- Patients who received aspiration could be randomized after the procedure if they were still symptomatic—It’s unclear how many patients, if any, were randomized after initial aspiration.
- It was the treating provider’s discretion to place a chest tube and admit or perform needle aspiration, then discharge.
- The ambulatory device could be placed at multiple locations, including the second ICS midclavicular or fifth ICS midaxillary. Could this have affected the function of the ambulatory device?
- The timing varied in how often patients were reviewed between the control and intervention groups. This could have contributed to the ambulatory group having the device in longer and greater numbers of serious adverse events.
- Patient selection may have also been affected based on the clinician’s skill, comfort level, and if patients presented outside the hours of ambulatory services.
Discussion:
Bias:
- This seems like a study trying to push a medical device. Why not just watch and wait? Use a nonrebreather flush rate O2 with repeat CXR at 4hrs instead of attempting an invasive procedure.
- Not all studies can be blinded, which may introduce reporting and confirmation bias. However, it’s critical to maintain homogeneity in management so that we may attribute any outcome difference to the intervention and not to management.
- Investigators allowed physician discretion to determine the time frame to maintain the treatment device. The ambulatory group had a longer treatment duration than the standard care group. Allowing providers to determine the duration of treatment could contribute to lower re-admission rates in the ambulatory group and introduces outcome bias and confirmation bias.
- Some eligible patients were not enrolled in the study if they presented outside the working hours of the National Health Services or if no trained staff members were available, which introduces selection bias.
Study Design:
- The ambulatory device is a variation of an 8-french pigtail catheter attached to a Heimlich valve secured with an adhesive. Rocket Medical UK supplied the device and paid multiple authors as consultants. Otherwise, it’s unclear what advantage the ambulatory device provides over the standard pigtail catheter commonly sutured in place.
- At its essence, the study compared outcomes in primary spontaneous pneumothorax when managed with a version of an 8 Fr pigtail catheter (ambulatory arm) vs. aspiration or small-bore chest drain < 14 Fr (standard arm).
- While aspiration may offer the benefit of decreased hospitalization, it is associated with more complications compared to tube thoracostomy for the management of pneumothorax (Carson-Chahhoud 2017).
- In addition, discharging a patient with pneumothorax may not be feasible for many clinicians. Many outpatient clinicians may not be comfortable managing chest drains. Likewise, the availability of a pneumothorax clinic may not be generalizable to all populations.
Conflicts of Interest:
- Rocket Medical UK provided the ambulatory pleural vent for this trial. Also, five authors received grants or consulting fees from Rocket Medical UK. In 2003 a systematic review and meta-analysis found studies funded by a company are four times more likely to have favorable results than studies from other sources (Lexchin 2003). Companies can also suppress negative studies leading to publication bias. Further, companies can design trials to get the desired results by conducting a trial against a product known to be inferior.
Authors’ Conclusion: “In patients with primary spontaneous pneumothorax, ambulatory management significantly reduces the duration of hospital stay during 30 days. Outpatient management is now a reasonable and probably preferable option in the management of this condition.”
Clinical Bottom Line
The increased risk of serious adverse events and the need for repeated close outpatient follow-up by a clinician trained to manage pneumothoraces and chest drains in the ambulatory cohort seem too much to overcome for most patients and clinicians. However, it may be a viable option for some with careful selection and shared decision-making. Perhaps a better strategy is to apply O2 and repeat CXR in 4 hours before attempting any invasive procedure.
References:
- Rochon PA, Gurwitz JH, Simms RW, Fortin PR, Felson DT, et al. (1994) A study of manufacturer-supported trials of nonsteroidal anti-inflammatory drugs in the treatment of arthritis. Arch Intern Med 154: 157–163. PMID: 8285810
- Smith R (2005) Medical Journals Are an Extension of the Marketing Arm of Pharmaceutical Companies. PLoS Med 2(5): e138. PMID: 15916457
- Yaphe J, Edman R, Knishkowy B, Herman J. The association between funding by commercial interests and study outcome in randomized controlled drug trials. Fam Pract. 2001 Dec;18(6):565-8. PMID: 11739337
- Carson-Chahhoud KV, Wakai A, van Agteren JEM, Smith BJ, McCabe G, Brinn MP, O’Sullivan R. Simple aspiration versus intercostal tube drainage for primary spontaneous pneumothorax in adults. Cochrane Database of Systematic Reviews 2017, Issue 9. Art. No.: CD004479. PMID: 28881006
- Lexchin J, Bero LA, Djulbegovic B, Clark O. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. BMJ. 2003;326(7400):1167-1170. PMID: 12775614
- Brims FJ, Maskell NA. Ambulatory treatment in the management of pneumothorax: a systematic review of the literature. Thorax. 2013 Jul;68(7):664-9. doi: 10.1136/thoraxjnl-2012-202875. Epub 2013 Mar 20. PMID: 23515437.
Guest Post By:

Lamisa Quaim, DO
PGY-2, Emergency Medicine Resident
Vassar Brothers Hospital, Poughkeepsie, New York
E-mail: Lamisa.quaim@nuvancehealth.org

Marco Propersi, DO FAAEM
Vice-Chair, Emergency Medicine
Vassar Brothers Hospital, Poughkeepsie, New York
Twitter: @marco_propersi
Post-Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The RAMPP Trial: Randomised Ambulatory Management of Primary Pneumothorax appeared first on REBEL EM - Emergency Medicine Blog.
