

Essentials of Emergency Medicine 2019 is taking place at the Cosmopolitan Hotel/Casino in Las Vegas, NV. I was asked to give five lectures on varying topics and wanted to share what I discussed at each of these sessions. If you haven’t been to Essentials of Emergency Medicine, you need to add this conference to your list of conferences to attend. The organizers pride themselves in discussing the latest practice-changing research and have meticulously designed content to maximize enjoyment and retention. In my humble opinion this conference is the quintessential medutainment extravaganza that applies learning theory principles, with amazing speakers, to provide you with the latest and greatest for clinical practice.
The Literature Behind the Rule: HEART Score
[embedyt] https://www.youtube.com/watch?v=OauE5w1jpY0[/embedyt]
Chest Pain (CP) is a very common complaint seen in emergency departments around the world. In the US specifically anywhere from 8 – 10 million patients present to the ED complaining of CP. Many use liberal testing strategies to prevent missing acute coronary syndrome (ACS) or other major adverse cardiac events (MACE), but this is not without increase in healthcare cost and false positive testing leading to more downstream testing. In recent years there have been several diagnostic protocols developed to help determine a portion of these patients as low risk to facilitate early discharge, prevent this over testing, while still having a >99% NPV for MACE at 30 days to 6 weeks. (Disclaimer: MACE = Composite of AMI, PCI/CABG, and death).
A recent meta-analysis was published in Ann Emerg Med Feb 2019 [1], which is summarized below:
- 25 studies
- ≈25k patients
- ≈40% Low Risk (HEART 0 – 3)
- 1% MACE Rate at 30d – 6weeks
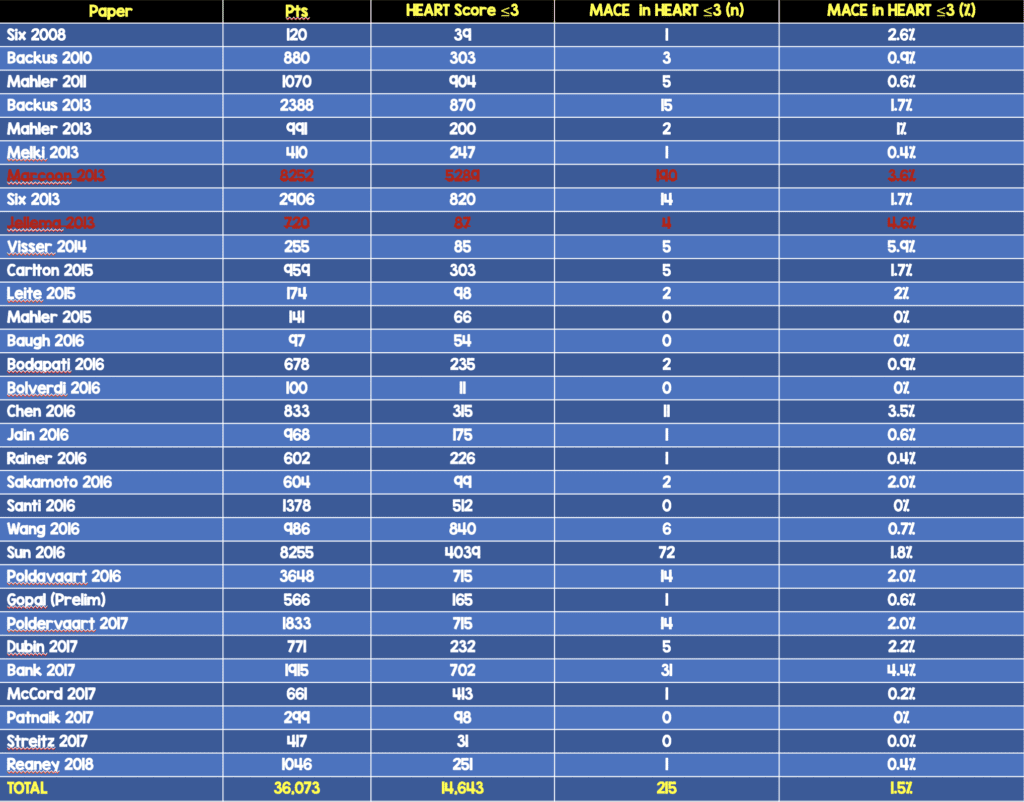
Upon reviewing this data, 6 trials with >14,000 patients were left out of the analysis. If those trials are added to the overall pool of studies, the results change slightly:
- 31 trials
- ≈38k patients
- ≈40% Low Risk (HEART 0 – 3) (ADDENDUM 8/29/2019: In the video I say 10%, but the number is actually 40%)
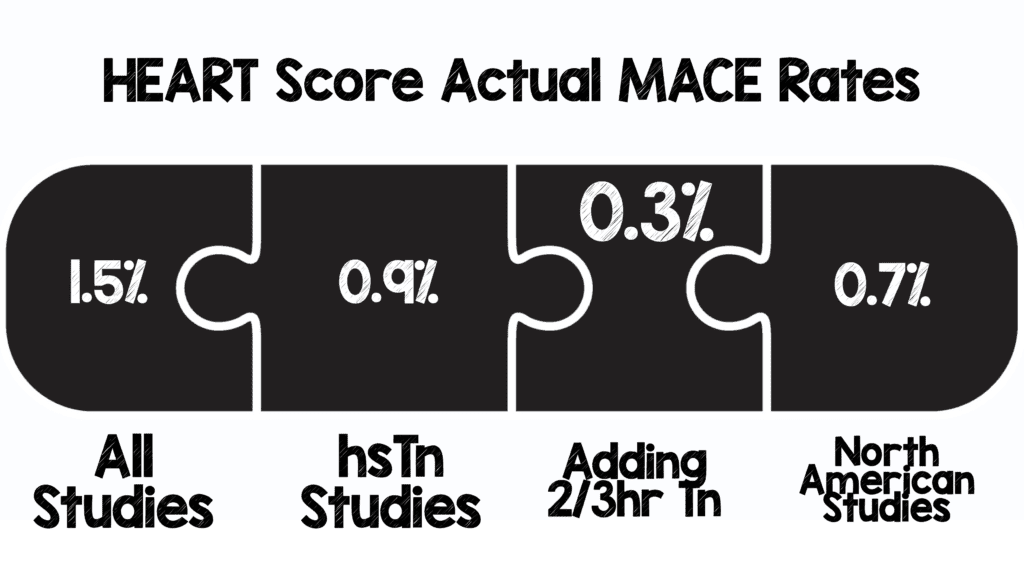
- 1.5% MACE Rate at 30d – 6weeks
To break down this data a bit further:
- For centers using high sensitivity troponin T or I:
- 7 studies
- ≈9k patients
- ≈40% identified as low risk
- 0.9% MACE Rate at 30d – 6weeks
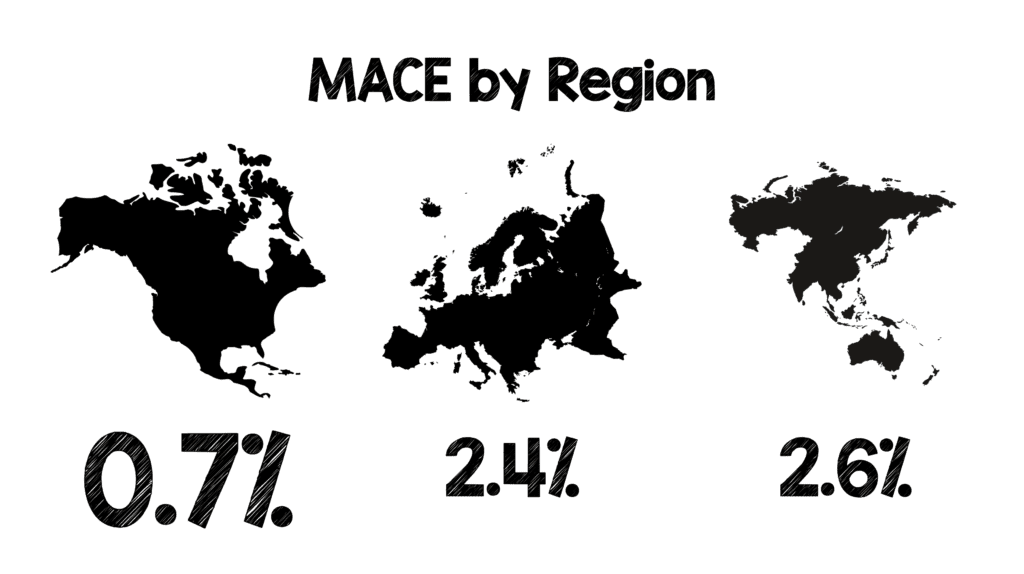
- Broken down by region of the world 9MACE Rate at 30d – 6weeks)
- North America: 0.7%
- Europe: 2.4%
- Asia-Pacific: 2.6%
Finally, the addition of the HEART Pathway (HEART Score ≤0 – 3 + 2/3hr Repeat Tn):
- 4 studies
- ≈2k patients
- 34.1% identified as low risk
- 0.3% MACE rate at 30d – 6 weeks
Bottom Line:
- Using the HEART score, MACE rate at 30d – 6weeks = 1.5%
- If you are using high-sensitivity troponin assays, MACE rate at 30d – 6weeks = 0.9%, also 40% of patients qualifying as low risk (same as contemporary troponin)
- We are not doing too bad in North America as application of the HEART score has yielded a MACE rate of 0.7% at 30d – 6weeks.
- Finally, the addition of the HEART Pathway, identifies about 1/3 of patients as low risk and decreases the 30d – 6week MACE rate down to 0.3%
References:
- Laureano-Phillips J et al. HEART Score Risk Stratification of Low-Risk Chest Pain Patients in the Emergency Department: A Systematic Review and Meta-Analysis. Ann Emerg Med Feb 2019. PMID: 30718010
The CENSER Trial: Early Norepinephrine in Septic Shock

Background: Standard management of septic shock has included, IV fluids until optimal intravascular volume is achieved, appropriate early antibiotics, and source control. Typically, only after all these measures have been undertaken is vasopressor infusion initiated if a MAP of ≥65mmHg is not achieved.
There have been some animal and human studies that have advocated for early norepinephrine administration in septic shock improving hemodynamics and mortality. The issue, with these trials is that they were retrospective which means these studies suffer from the limitations of this type of methodology (i.e. convenience sampling, recall bias, confounding, and ultimately cannot determine causation, only association).
What They Did:
- Prospective, single center, randomized, double-blind, placebo-controlled clinical trial evaluating the use of early low-dose norepinephrine (eNE) vs standard care (SC) (i.e placebo) in adults with septic shock to increase shock control by 6 hours in Bangkok, Thailand
- eNE = 4mg of norepinephrine mixed in 250mL of D5W = 16ug/mL
- SC (Placebo) = 250mL of D5W
Results:
- 310 adults diagnosed with septic shock
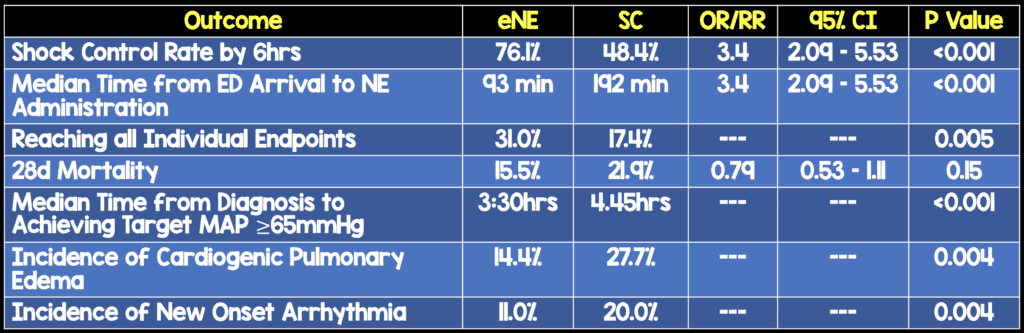
- No difference in number of patients admitted to ICU, amount of IV fluid received between groups
Discussion:
- The timing of intervention in this study as a median of 93 minutes. Just for comparison the median time to intervention in ProCESS, ARISE, and PROMISE was 162 minutes in the EGDT arm and 159 minutes in the standard care arm
- Although, secondary outcome of mortality was not statistically significant, it was clearly better in the eNE arm.
- Most if not all secondary endpoints were better in the eNE arm, but not statistically significant.As these are secondary endpoints, this would be hypothesis generating
- >50% of patients had vasopressors run through peripheral IV with only 1 case of skin necrosis (0.6%) in each arm of the study, which again confirms the use of vasopressors via peripheral IVs
- The authors of this paper appropriately state: “A multicenter trial with a larger population size, control of the rate of fluid resuscitation, and timing of norepinephrine initiation is certainly required to assess the survival benefit of early norepinephrine as an intervention.”
Clinical Take Home Point: Although, this study confirms my own biases of initiating vasopressor therapy earlier in the course of patients with septic shock, it should be remembered that this study still requires external validation with patient-oriented outcomes before implementation into routine clinical practice.
Currently in my practice, in patients with septic shock, I am starting with a Lactated Ringers bolus and assessing fluid status with RUSH exam. If my patient is euvolemic or hypervolemic, I am beginning my norepinephrine infusion at a much sooner time than waiting for 30cc/kg to be completed
References:
- Permpikul et al. Early Use of Norepinephrine in Septic Shock Resuscitation (CENSER): A Randomized Trial. AJRCCM 2019. [Epub Ahead of Print]
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post The REBEL at Essentials of Emergency Medicine 2019 – Day 1 appeared first on REBEL EM - Emergency Medicine Blog.
