 Background: We have been in need of a sign of hope in the fight against SARS-CoV-2 as it runs from city to city overwhelming health systems. The majority of patients will be either asymptomatic or have only mild disease. These patients will improve for the most part with symptomatic care. There is a smaller portion of patients admitted to the hospital and ICU requiring oxygen therapy or invasive mechanical ventilation (IMV). In this group of patients, there has not been much promise in the way of treatments improving mortality. Patients requiring oxygen therapy (HFNC, NIV, IMV, ECMO) are mostly in the pulmonary and hyperinflammatory stage of disease (see below figure). One theoretical option in this hyperinflammatory stage of disease is corticosteroids to help quell the immune response and potentially improve mortality outcomes.
Background: We have been in need of a sign of hope in the fight against SARS-CoV-2 as it runs from city to city overwhelming health systems. The majority of patients will be either asymptomatic or have only mild disease. These patients will improve for the most part with symptomatic care. There is a smaller portion of patients admitted to the hospital and ICU requiring oxygen therapy or invasive mechanical ventilation (IMV). In this group of patients, there has not been much promise in the way of treatments improving mortality. Patients requiring oxygen therapy (HFNC, NIV, IMV, ECMO) are mostly in the pulmonary and hyperinflammatory stage of disease (see below figure). One theoretical option in this hyperinflammatory stage of disease is corticosteroids to help quell the immune response and potentially improve mortality outcomes.
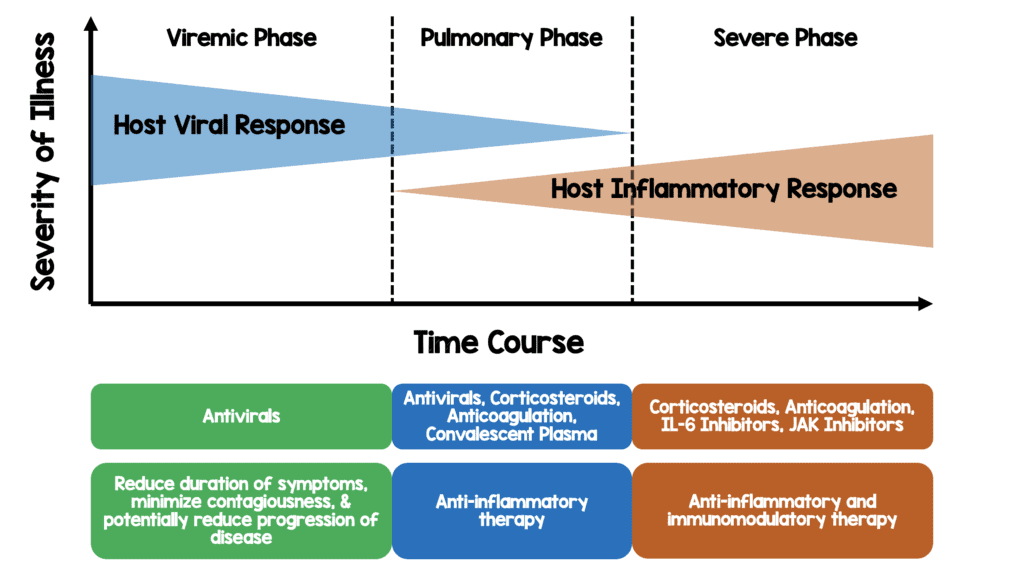
This is a theoretical model of disease progression with potential targeted therapies based on my reading of the literature thus far
Paper: Horby PW et al. Effect of Dexamethasone in Hospitalized Patients With COVID-19 – Preliminary Report. medRxiv Preprint 2020. [Epub Ahead of Print]
Clinical Question: Does dexamethasone (IV or PO) + usual care reduce 28 day mortality in patients hospitalized with COVID-19 compared to usual care alone?
What They Did:
- Randomized Evaluation of COVID-19 Therapy (RECOVERY)
- Randomized, controlled, open-label, adaptive, platform trial performed at 176 hospitals in the United Kingdom
- Patient randomized in a 2:1 ratio (standard care vs intervention respectively)
- Platform trial compares a range of possible treatments with usual care in patients hospitalized with COVID-19 (i.e. multiple arms of the same study with different groups of patients randomized to a treatment vs usual care)
- Preliminary results of:
- Dexamethasone 6mg (PO or IV) qD for up to 10d
- Usual care alone
Outcomes:
- Primary: 28d Mortality
- Prespecified subgroup analysis of those patients on O2, IMV, and those getting neither
- Secondary:
- Time to discharge from hospital and among patients not receiving IMV at randomization
- Subsequent receipt of IMV (Including ECMO) or death
- Cause-specific mortality
- Receipt of renal hemodialysis or hemofiltration
- Major cardiac arrhythmia
- Receipt and duration of ventilation
Inclusion:
- Clinically suspected or laboratory confirmed SARS-CoV-2 infection
- No medical history that might, in the opinion of the attending clinician, put the patient at significant risk if they were to participate in the trail
- Initially ≥18 years of age (age limit removed on May 9th, 2020)
- Pregnant or breast-feeding women were eligible
Exclusion:
- Nothing stated in manuscript (Patients being discharged home)
Results:
- 6425 patients randomized
- Dexamethasone: 2104 patients
- Usual Care: 4321 patients
- At Randomization:
- IMV/ECMO: 16.0%
- O2 Therapy: 60%
- No O2: 24.0%
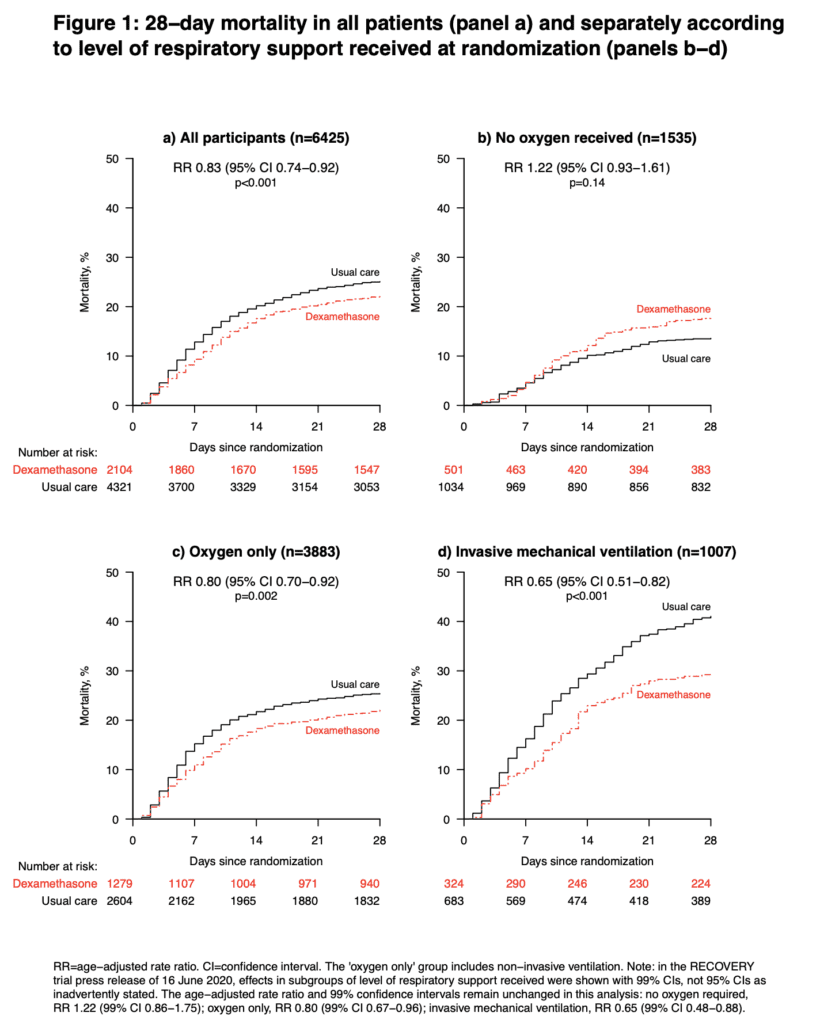
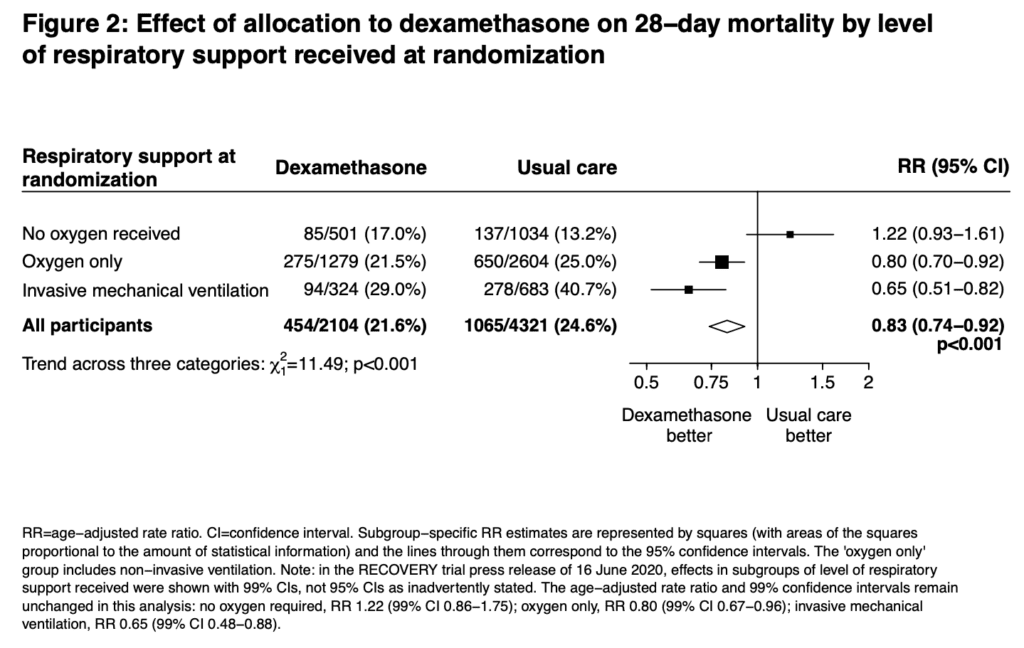
Strengths:
- Large, pragmatic, randomized controlled trial
- Use an adaptive platform trial design to provide rapid and robust assessment of the impact of readily available potential treatments for COVID-19 on 28-day mortality
- Follow up information was complete for 95% of randomized patients
- Of the patients randomized to dexamethasone, 95% received at least one dose
Limitations:
- Several patients were excluded as either dexamethasone was either unavailable at the hospital at the time of enrollment or considered by the managing doctor to be contraindicated. It is unclear how many were excluded from this analysis and how this would affect the results of the study (It appears about 17% from the results section, but not explicitly stated)
- Open label study, means everyone knew which arm of the study they were enrolled in, which could bias the results (although mortality is an objective outcome and difficult to bias if someone is dead or alive). Thinking in terms of the amount of care provided to a group getting steroids being higher quality than patients not getting steroids could be one way to bias this result
Discussion:
- By random chance the patients in the dexamethasone arm were 1.1 years older than the patients in the usual care arm. The authors accounted for this imbalance with adjustment for baseline age. The adjustment was not specified in the first version of the statistical analysis plan and added once the imbalance in age became apparent. The authors give results with and without age-adjustment and showed it did not alter the conclusions
- A sample size could not be estimated for this trial, but a steering committee, blinded to the results of the study formed a “best guess” of 2000 vs 4000 patients with an estimated 20% 28d mortality could detect an absolute difference of 4% between the two groups
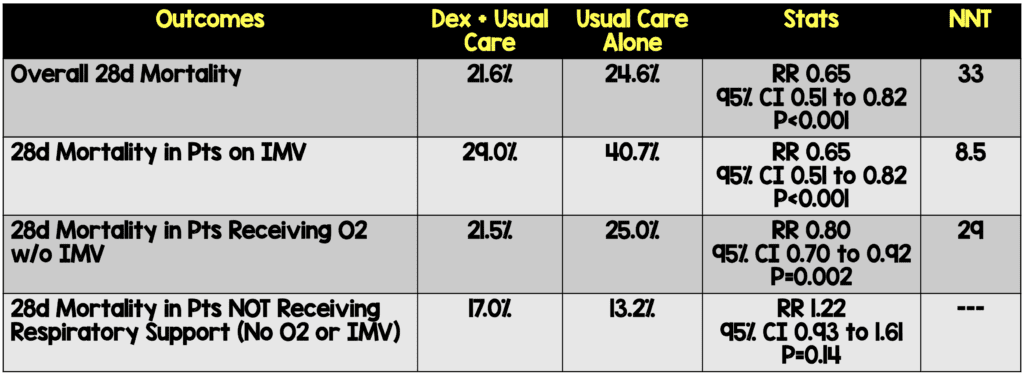
- 28-day mortality in the usual care group was highest in those patients receiving IMV (40.7%), intermediate in those receiving oxygen only (25.0%), and lowest among those who were not receiving respiratory support at randomization (13.2%). The greatest absolute reductions in 28-day mortality were seen in the sickest patients
- The preliminary results for dexamethasone were announced on June 16th, 2020, just 98 days after the protocol was first drafted. This is amazingly fast!!!
- When looking at one of the pre-specified baseline characteristics, it appears that patients with >7d of symptoms did better than patients ≤7d. The timing of dexamethasone in this disease may be an important factor to consider
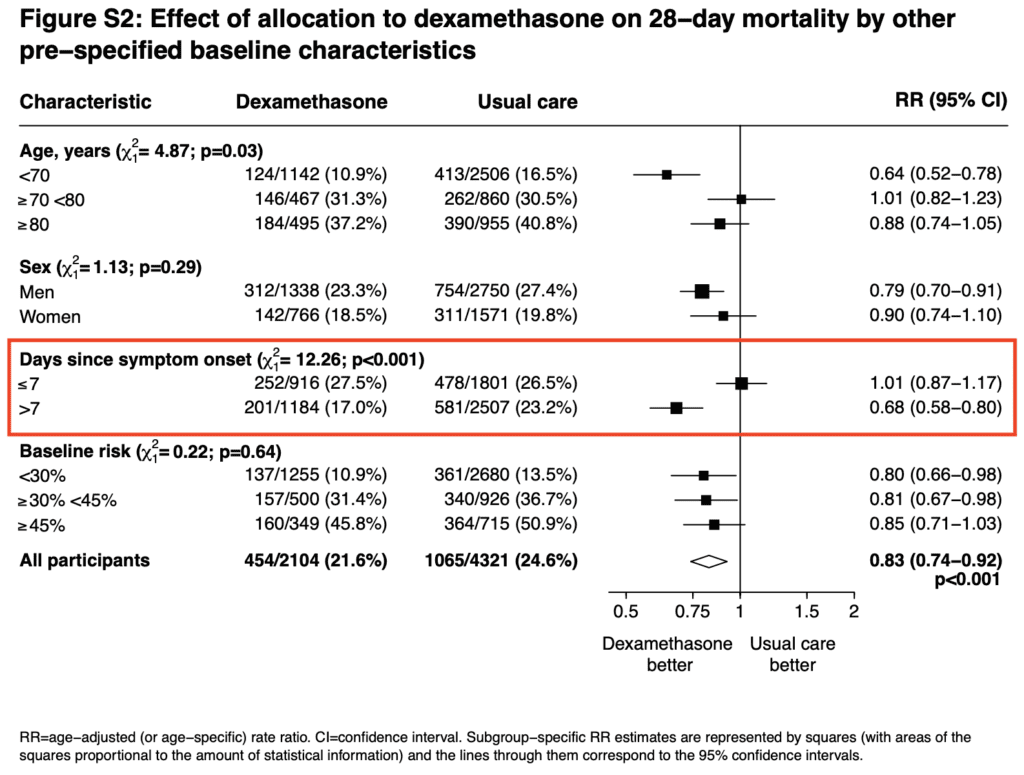
- My thoughts on the reason why those not on O2 had a signal for worse outcome is because they’re in the viral phase of illness and immunosuppression will likely worsen disease in this phase (i.e. immunosuppressing too early in illness could potentially cause worsened outcomes)
Author Conclusion: “In patients hospitalized with COVID-19, dexamethasone reduced 28-day mortality among those receiving invasive mechanical ventilation or oxygen at randomization but not among patients not receiving respiratory support.”
Clinical Take Home Point: The findings of this well done randomized controlled trial indicate that in patients with COVID-19 pneumonia, that dexamethasone improves 28d mortality compared to placebo in patients requiring IMV (NNT = 8.5) and those patients requiring oxygen therapy (NNT = 29). There was no benefit to patients not requiring oxygenation support and even a signal for harm. It is important to remember this is a preprint, preliminary report, which is better than a press release. At this time, in patients requiring any oxygen therapy, HFNC, NIV, IMV, or ECMO I would recommend dexamethasone until further evidence disproves this benefit.
References:
- Horby PW et al. Effect of Dexamethasone in Hospitalized Patients With COVID-19 – Preliminary Report. medRxiv Preprint 2020. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- FOAMCast: Dexamethasone [RECOVERY Trial]
- Broome Docs: CoVid, Dex and Death…Recovery
- PulmCrit: Dexamethasone & COVID – A Stud in Immunopathology, Evidence-Based Medicine, and Ourselves
- First10EM: Dexamethasone for COVID – The RECOVERY Trial
- St. Emlyn’s: Dexamethasone, COVID-19, and the RECOVERY Trial
- The Bottom Line: RECOVERY – COVID-19 Dexamethasone
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post The RECOVERY Trial: Dexamethasone for COVID-19? appeared first on REBEL EM - Emergency Medicine Blog.