 Background: Droperidol came onto the market in 1967 and, over time, became a frequently employed treatment of headache, nausea, agitation, acute pain, chronic pain, pain in the context of opioid-tolerance and refractory abdominal pain. Unfortunately, in December 2011, the US Food and Drug Administration placed a black box on droperidol due to surveillance data showing increased prevalence of QT prolongation. Most of these cases were due to high doses of droperidol ranging from 50 to 100 mgs. It’s also important to point out that QTc prolongation is not a patient centered outcome. A trial published by Nuttall et al of over 20,000 patients found ZERO cases of polymorphic VT or death at low doses of droperidol (0.625mg) [3]. Additionally, the Clinical Guidelines Committee of the American Academy of Emergency Medicine (AAEM) reviewed the literature in 2014 and found no evidence that low dose droperidol (under 2.5mg) was unsafe for use [4].
Background: Droperidol came onto the market in 1967 and, over time, became a frequently employed treatment of headache, nausea, agitation, acute pain, chronic pain, pain in the context of opioid-tolerance and refractory abdominal pain. Unfortunately, in December 2011, the US Food and Drug Administration placed a black box on droperidol due to surveillance data showing increased prevalence of QT prolongation. Most of these cases were due to high doses of droperidol ranging from 50 to 100 mgs. It’s also important to point out that QTc prolongation is not a patient centered outcome. A trial published by Nuttall et al of over 20,000 patients found ZERO cases of polymorphic VT or death at low doses of droperidol (0.625mg) [3]. Additionally, the Clinical Guidelines Committee of the American Academy of Emergency Medicine (AAEM) reviewed the literature in 2014 and found no evidence that low dose droperidol (under 2.5mg) was unsafe for use [4].
Paper: Gaw CM et al. Effectiveness and Safety of Droperidol in a United States Emergency Department. AJEM 2020. PMID: 31831345 [Access on Read by QxMD]
Clinical Question: What is the effectiveness and safety of droperidol used in patients presenting to the ED?
What They Did:
- Retrospective, observational, cohort study
- All droperidol administrations from 1/1/20212 to 04/18/2018 at a single ED in the US
- Mortality analysis included all adults and children who received droperidol in the ED
- All other results and analyses only included patients who gave consent
Outcomes:
- Primary: 24hr Mortality
-
Secondary:
- Use of rescue analgesics (Another medication for pain or headache in 30 to 60 minutes after the first administration of droperidol)
- Extrapyramidal symptoms
Inclusion:
- Administration of droperidol in the ED
- Consent to research use of records
- ECG data, medical record reports of comorbidities, and PMH available
Exclusion:
- None stated
Results:
- 6,881 visits in which droperidol administered to 5,784 distinct pts
- 6,353 visits by 5,334 distinct patients authorized use of their records
- Administration in children = 5.4%
- Administration in older adults (≥65yrs) = 8.2%
- Median age = 38 years (Range: 28 to 51)
- Indications for droperidol use:
- Analgesic for pain = 21.8%
- Headache = 57.0%
- Sedative = 8.7%
- Antiemetic = 12.5%
- ZERO deaths within 24hrs secondary to droperidol administration
- ZERO fatal arrhythmias secondary to droperidol administration
- Need for rescue analgesia:
- Pts with headache = 5.2%
- Rescue opioids = 1.1%
- Pts with pain other than headache = 7.4%
- Rescue opioids = 5.4%
- 796 patients had charts reviewed and had complete symptom resolution with droperidol:
- Headache: 79.5%
- Pain other than headache: 50.0%
- Nausea/vomiting: 56.5%
- Behavioral sedation: 48.3%
- Zero cases of akathisia or dystonia coded in electronic data set
- However, in the 796 independent chart reviews, akathisia occurred in 2.9% of patients
- All cases were resolved with diphenhydramine
-
QTc on ECG 24 hours after droperidol:
- No = 4,679 (73.7%)
- Yes, with QTc <500 = 1,631 (25.7%)
- Yes, with QTc ≥500 = 43 (0.7%)
- Of the 43 pts with QTc ≥500, 25 of them had ECG 6 months prior of which 8 had an ECG showing a long QTc
-
Median dose of droperidol used in 796 individual charts reviewed = 0.625mg
- 0.625mg = 52.1%
- ≥2.5mg = 40.5%
- 5mg = 2.4%
- Pts with headache = 5.2%
- 6,353 visits by 5,334 distinct patients authorized use of their records
Strengths:
- Study adhered to the STROBE (Strengthening the reporting of Observational studies in Epidemiology) guidelines for reporting observational studies
- To minimize risk of bias the authors calculated interrater agreement with the electronic chart review
- Mortality data was obtained for all patients
Limitations:
- Complete symptom resolution only evaluated in a small percentage of the overall study population (i.e. 796 out of 6,353 droperidol dosages)
- Single center, retrospective study may not be generalizable to other health systems
- Retrospective nature of trial makes it dependent on the accuracy and availability of data in the medical record
- Observational nature suggests an association of effectiveness of droperidol as an analgesic, antiemetic, and sedative but does not give causation to its efficacy
- Outcomes extracted by a single abstractor not blinded to the study hypothesis
- Adverse events of akathisia and dystonia not recorded as a final diagnosis in any of the patients identified through medical record review as having an adverse effect. Most likely these were underreported
Discussion:
- Among 1,674 pts who had an ECG obtained within 24hrs of droperidol administration, there were no clinically significant arrhythmias or deaths attributable to droperidol itself.
- The only death within 24hrs after droperidol was secondary to respiratory failure in a patient with metastatic malignancy
- The only adverse effect noted was akathisia which was infrequent (2.9% of reviewed charts) and transient (all resolved with diphenhydramine)
-
Previous Evidence:
- The AAEM study from 2014 [4] which reviewed trials prior to 2015, found no evidence that low dose droperidol (<2.5mg) was unsafe for use in the ED
- Incidence of Torsades des Pointes Since 2015:
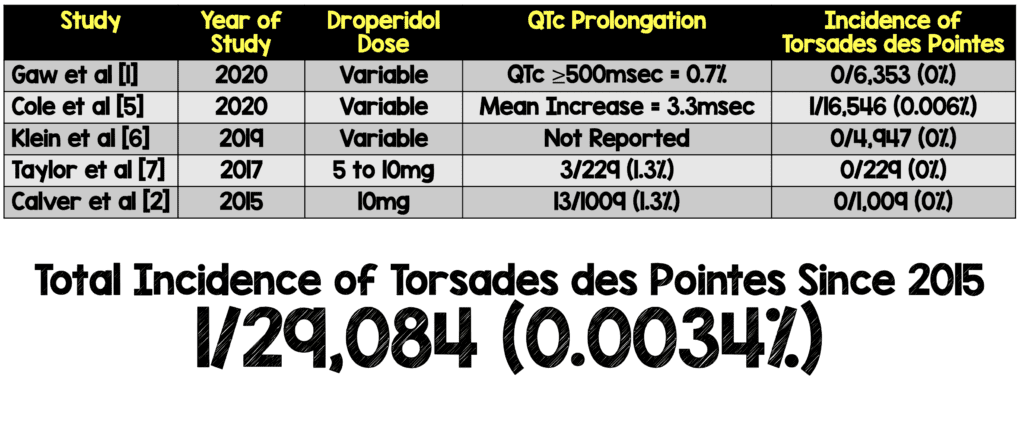
- According to the authors acute QTc prolongation is short-lived with droperidol (peaking at 10 min and disappearing at 15 min)
- Droperidol vs Haldoperidol:
- Droperidol
- Onset of Action: 5 to 10min (a bit faster with IV)
- Peak Effect: 20 to 30min (a bit faster with IV)
- Duration of Action: 2 to 4hrs
- Haloperidol
- Onset of Action: 20 to 30min (a bit faster with IV)
- Peak Effect: 30 to 60min (a bit faster with IV)
- Duration of Action: 6 to 20hrs
Author Conclusion: “No fatalities were seen among this large cohort of patients who received droperidol in the ED. Our findings suggest droperidol’s effectiveness and safety when used as an analgesic, antiemetic and/or sedative.”
Clinical Take Home Point: In this large, retrospective, observational data set from a single hospital, the use of droperidol was not associated with any clinically significant arrhythmias or mortality within 24 hours of administration. Additionally, droperidol was found to be effective for the treatment of pain, nausea/vomiting, and as a sedative agent.
References:
- Gaw CM et al. Effectiveness and Safety of Droperidol in a United States Emergency Department. AJEM 2020. PMID: 31831345 [Access on Read by QxMD]
- Calver L et al. The Safety and Effectiveness of Droperidol for Sedation of Acute Behavioral Disturbance in the Emergency Department. Ann Emerg Med 2015. PMID: 25890395 [Access on Read by QxMD]
- Nuttall GA et al. Does Low-Dose Droperidol Increase the Risk of Polymorphic Ventricular Tachycardia or Death in the Surgical Patient? Anesthesiology 2013. PMID: 23291623
- Perkins J et al. American Academy of Emergency Medicine Position Statement: Safety of Droperidol Use in the Emergency Department. JEM 2015. PMID: 25837231
- Cole JB et al. The Incidence of QT Prolongation and Torsades des Pointes in Patients Receiving Droperidol in an Urban Emergency Department. WJEM 2020. PMID: 32726229
- Klein LR et al. Rescue Sedation When Treating Acute Agitation in the Emergency Department with Intramuscular Antipsychotics. JEM 2019. PMID: 30745194
- Taylor DM et al. Midazolam-Droperidol, Droperidol, or Olanzapine for Acute Agitation: A Randomized Clinical Trial. Ann Emerg Med 2017. PMID: 27745766
For More on This Topic Checkout:
- REBEL EM: Droperidol Reigns Supreme
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post Droperidol: Making a Comeback!!! appeared first on REBEL EM - Emergency Medicine Blog.

