
 Background: The CRASH-2 trial, published in 2010, showed a survival benefit for patients with traumatic hemorrhage who received TXA compared to placebo. TXA has become standard practice in many settings as a result of this data. However, patients with significant head injury were excluded in this study and, it was unclear of the effect of TXA in this group. In 2019, CRASH-3 examined the use of TXA in traumatic brain injury (TBI) and found the overall effect size of TXA on ICH was not statistically significant compared to placebo. Subgroup analysis demonstrated that certain patients (<3hrs, GCS 9 – 15, and ICH on baseline CT) showed benefit with TXA. However, this data was hypothesis generating only: not the primary outcome of the trial and with wide confidence intervals. Further data is clearly needed to elucidate the role of TXA in those with TBI.
Background: The CRASH-2 trial, published in 2010, showed a survival benefit for patients with traumatic hemorrhage who received TXA compared to placebo. TXA has become standard practice in many settings as a result of this data. However, patients with significant head injury were excluded in this study and, it was unclear of the effect of TXA in this group. In 2019, CRASH-3 examined the use of TXA in traumatic brain injury (TBI) and found the overall effect size of TXA on ICH was not statistically significant compared to placebo. Subgroup analysis demonstrated that certain patients (<3hrs, GCS 9 – 15, and ICH on baseline CT) showed benefit with TXA. However, this data was hypothesis generating only: not the primary outcome of the trial and with wide confidence intervals. Further data is clearly needed to elucidate the role of TXA in those with TBI.
Paper: Rowell SE et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury. JAMA 2020. [Epub Ahead of Print]
Clinical Question: Does early administration of TXA to patients with moderate or severe traumatic brain injury improve neurologic outcomes at 6 months?
What They Did:
- Randomized, multicenter, double-blinded, 3-group, phase II, clinical trial in the out-of-hospital setting within 2 hours of injury
- 12 sites, 20 trauma centers, and 39 EMS service agencies in the US and Canada
- Randomized to:
- Bolus Maintenance Group: Out-of-hospital TXA 1g bolus and in-hospital TXA 1g 8-hour infusion
- Bolus Only Group: Out-of-hospital TXA 2g bolus and in-hospital placebo 8-hour infusion
- Placebo Group: Out-of-hospital placebo bolus and in-hospital placebo 8-hour infusion
Outcomes:
-
Primary: Favorable neurologic function at 6 months
- Glasgow Outcome Scale-Extended score >4 = moderate disability or good recovery
- Significance thresholds set at 0.1 for benefit and 0.025 for harm
-
Secondary:
- 18 secondary endpoints, of which 5 reported statistical analysis in this article
- 28-day mortality
- 6-month Disability Rating Scale score
- 0 = No disability
- 30 = Death
- Progression of ICH (defined as >33% increase in the combined volume)
- Incidence of seizures
- Incidence of thromboembolic events
Inclusion:
- Age ≥15 years
- Moderate or severe blunt or penetrating TBI (GCS 3 to 12)
- At least one reactive pupil
- SBP of at least 90mmHg prior to randomization
- Intravenous catheter in place and study drug could be administered within 2 hours of injury
- Predefined EMS transport destination was a participating trauma center
Exclusion:
- Prehospital GCS = 3 with no reactive pupil
- Estimated time from injury to start of study drug bolus >2hrs
- Patients with SBP <90mmHg prior to randomization
- Unknown time of injury
- Clinical suspicion of seizure activity, acute MI, or stroke, or known history, to the extent possible of seizure, thromboembolic disorders or renal dialysis
- CPR by EMS prior to randomization
- Burns >20% total body surface area
- Suspected or known prisoners
- Suspected or known pregnancy
- Prehospital TXA or another pro-coagulant drug given prior to randomization
Results:
- 1063 patients were randomized
- 966 participants in the analysis population
- Mean GCS score = 8
- Mean time from injury to out-of-hospital study drug administration ranged from 40 to 43min across groups
- Overall all-cause 28 mortality = 16% (of which 87% was attributed to neurologic injury)
- 819 (84.8%) of participants available for primary outcome analysis at 6-month follow up
- Favorable Neurologic Function at 6 Months:
- TXA groups: 65%
- Placebo group: 62%
- Difference -3.5%
- Not statistically significant
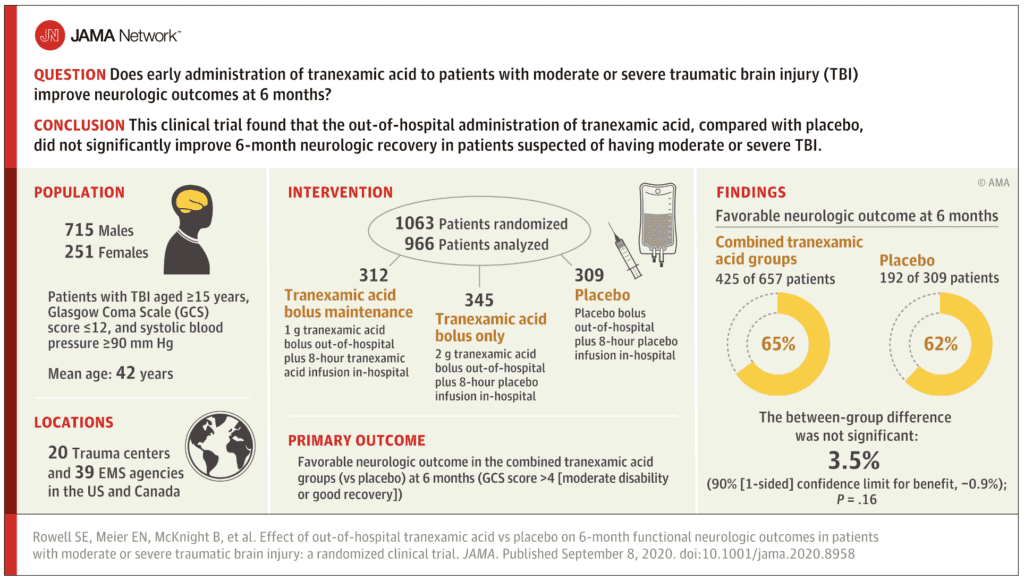
- No statistically significant difference in 28-day mortality between groups (14% vs 17%), Disability Rating Scale score (6.8 vs 7.6), or progression of ICH (16% vs 20%)
- 28-day mortality:
- Bolus Only: 12.0%
- Bolus Maintenance: 17.0%
- Adjusted Difference for Bolus Maintenance vs Placebo: -0.09%; 95% CI -6.1% to 5.9%; p = 0.98
- Adjusted Difference for Bolus Only vs Placebo: -5.4%; 95% CI -10.9% to 0.05%; p = 0.05
- Adjusted Difference for Bolus Only vs Bolus Maintenance: -5.3%; 95% CI -10.8% to 0.1%; p = 0.06
- Adverse Events:
- Thrombotic Events:
- Bolus Only: 9%
- Bolus Maintenance: 4%
- Placebo: 10%
- Seizures:
- Bolus Only: 5%
- Bolus Maintenance: 2%
- Placebo: 2%
- Thrombotic Events:
Strengths:
- Multicenter, randomized, double-blind, clinical trial
- Mean time from injury to study drug was short (i.e. ≈40 minutes)
- Group assignments were blinded to all EMS agencies, pharmacists, coordinators, and providers throughout the study
- Chose doses of TXA based on prior evidence and an alternative dosing regimen that could be more feasible in the prehospital and military settings
- Groups well balanced with respect to demographics and baseline anatomic/physiologic characteristics (with the exception of fewer penetrating injuries in the bolus only group)
- Injury severity similar between groups based on GCS score, ISS, and AIS head score
Limitations:
- Initial design of trial was to compare each of the 2 TXA dosing regimens separately with placebo, but instead a comparison of the combined TXA groups with placebo was performed
- 345 patients out of 1280 (26.9%) eligible patients were not enrolled due to TXA kit not being opened
- Emergency unblinding occurred in 3% of patients, of whom 53% received open-label TXA
- Bolus completion ranged from 93 to 95% and the in-hospital dose completion percentage was much lower ranging from 69 to 77%
- Not enough adverse events to draw definitive conclusions on the harms of TXA from this trial alone
- Early mortality difference observed between treatment groups has the potential to lead to survival bias (severely injured patients who survive longer in the bolus only group may have lived long enough to experience more complications)
- 20% of patients enrolled in the trial had a GCS score of 13 or higher on admission. This could dilute the overall injury severity biasing the results to show no difference in outcomes (i.e. higher GCS score means, more likely to recover regardless of treatment)
- 15% of patients were lost to follow up due to study withdrawal or inability to locate patients
- Heterogeneity may potentially exist due to the enrollment of blunt and penetrating injuries (Only 3% of patients in this trial experienced penetrating injury)
- Several secondary outcomes compared small numbers of patients, which could lead to false results
Discussion:
- There are now 2 RCTs examining the use of TXA in patients with TBI, both of which demonstrated no statistically significant differences in their primary outcomes comparing TXA vs placebo
- This current trial did not examine patients with less severe injury, but the CRASH-3 trial did. In the CRASH-3 trial there was a significantly lower risk of head injury-related death in less severely injured patients (mild to moderate TBI) who received TXA vs placebo
- This trial examined 2 different dosing regimens of TXA and performed exploratory analyses on a subset of patients with ICH. No statistically significant difference in ICH progression was observed between patients who received TXA vs placebo in this trial
- Using GCS score as a measure of severity of injury has limited ability to discriminate between ICH and other CNS depressed states (i.e. intoxication, sedation, shock). In this trial a small number of patients were enrolled that had ICH which could dilute treatment differences
- Adverse events, such as seizures with the use of TXA have been documented in previous trials. Although there was no statistically significant difference in the rate of seizures, there was a trend toward increased seizures with higher TXA doses (i.e. bolus only group) compared to the other to regimens. Too few patients experienced any adverse events to draw meaningful conclusions on these harms
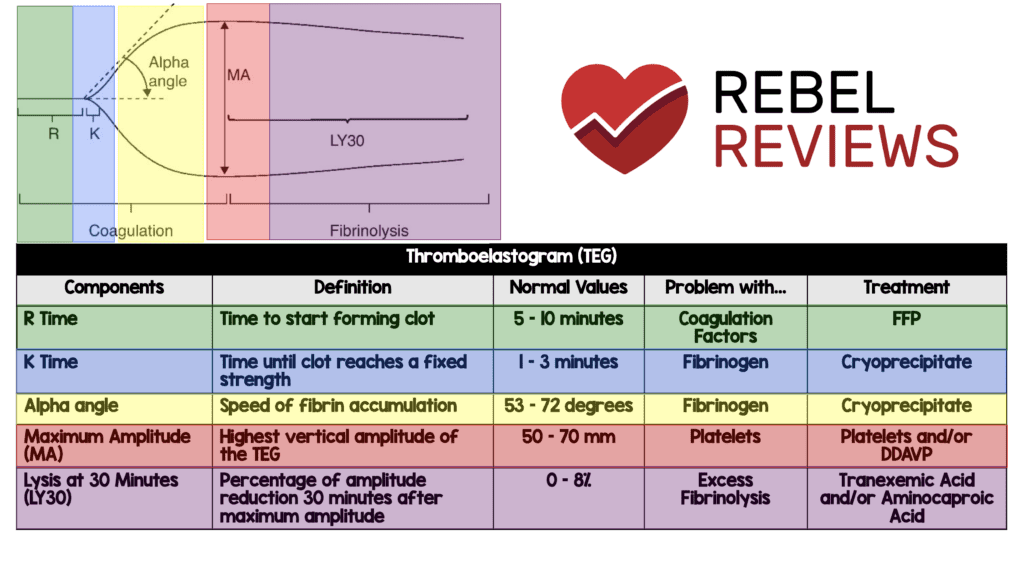
- TEG was drawn at admission in about 80 patients per group. When looking at these patients, the portion of the TEG we would expect to see a difference is in the Lysis at 30 Minutes (LY30) where excess fibrinolysis occurs. The normal range for this is 0 to 8%. In this trial the patients who had TEGs drawn we see a lower median number in the range in all groups. We would expect this to be lower in the TXA groups (as TXA inhibits breakdown of fibrin clot) than the placebo groups:
- Bolus Maintenance: 0.3 (Range 0 – 1.2)
- Bolus Only: 0.4 (Range 0 – 1.4)
- Placebo: 0.4 (Range 0 to 1.4)
- Mortality rates in this trial and CRASH-3 were fairly similar (≈20%), but patients in this trial had more severe brain injury compared to CRASH-3 (GCS 13 to 15: 3% in current trial vs 28% in CRASH-3).
- A minimal clinically important difference in head injury is a crucial question that remains unanswered. In an accompanying editorial [2] the authors flush out this point. For example, a 1% difference could decrease mortality by ≈560 deaths per year in the US. If we use the 2.9% difference in this study, that would decrease mortality by 1600 deaths per year in the US.
- A 3.5% difference in outcome was not statistically significant but, CRASH 2 was a 1.5% mortality benefit. It is very likely that the real difference is smaller than 3.5% but still clinically important and, would be statistically significant different with a larger group of patients. Even a 1% reduction in mortality would be clinically important for a drug without side effects
- Like CRASH3, this trial tells us that it’s unlikely TXA harms those with TBI so, if you have TBI and hemorrhagic shock, give TXA
- TXA was administered to a large number of patients who had no potential for benefit (409pts or 43%) with no/minimal brain injury or nonsurvivable injury. Additionally, only 545 patients (56%) had ICH on CT scan that would potentially benefit from TXA with the remainder of patients potentially diluting the results
Author Conclusion: “Among patients with moderate to severe TBI, out-of-hospital tranexamic acid administration within 2 hours of injury compared with placebo did not significantly improve 6-month neurologic outcome as measured by the Glasgow Outcome Scale-Extended.”
Clinical Take Home Point: This trial of TXA vs placebo given within 2 hours of injury, in the out-of-hospital setting, in patients with moderate or severe traumatic brain injury (GCS ≤12) combined with the results of the CRASH-3 trial give us no definitive evidence to support the routine use of out-of-hospital TXA in TBI. Although there is no benefit in moderate to severe TBI based on this trial, if a patient has concomitant head injury and hemorrhagic shock, TXA should be given as both this trial and CRASH-3 show minimal to no harm.
References:
- Rowell SE et al. Effect of Out-of-Hospital Tranexamic Acid vs Placebo on 6-Month Functional Neurologic Outcomes in Patients With Moderate or Severe Traumatic Brain Injury. JAMA 2020. [Epub Ahead of Print]
- Cone DC et al. Out-of-Hospital Tranxamic Acid for Traumatic Brain Injury. JAMA 2020. [Epub Ahead of Print]
For More Thoughts on This Topic Checkout:
- EM Lit of Note: TXA, The Miracle Drug With Mostly Negative Trials
- REBEL EM: CRASH-3 – TXA for ICH?
- St. Emlyn’s: TXA in Severe Head Injury
- First10EM: TXA in Traumatic Brain Injury – A Quick Update
- The SGEM: SGEM#305 – Somebody Get me a Doctor – But do I Need TXA by EMS for a TBI?
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post Out-Of-Hospital TXA for TBI appeared first on REBEL EM - Emergency Medicine Blog.


