
 Background: Convulsive status epilepticus is the most common pediatric neurological emergency worldwide. Currently, phenytoin (UK & Europe) or fosphenytoin (USA) is the recommended second-line IV anticonvulsant for the treatment of pediatric status epilepticus. Some evidence and providers however suggest that levetiracetam could be an effective and safer alternative. Recently not one, but two RCTs were published trying to figure out whether levetiracetam or phenytoin should be second-line treatment of pediatric status epilepticus.
Background: Convulsive status epilepticus is the most common pediatric neurological emergency worldwide. Currently, phenytoin (UK & Europe) or fosphenytoin (USA) is the recommended second-line IV anticonvulsant for the treatment of pediatric status epilepticus. Some evidence and providers however suggest that levetiracetam could be an effective and safer alternative. Recently not one, but two RCTs were published trying to figure out whether levetiracetam or phenytoin should be second-line treatment of pediatric status epilepticus.
REBEL Cast Episode 71: 2nd Line Therapy for Pediatric Status Epilepticus – EcLiPSE & ConSEPT
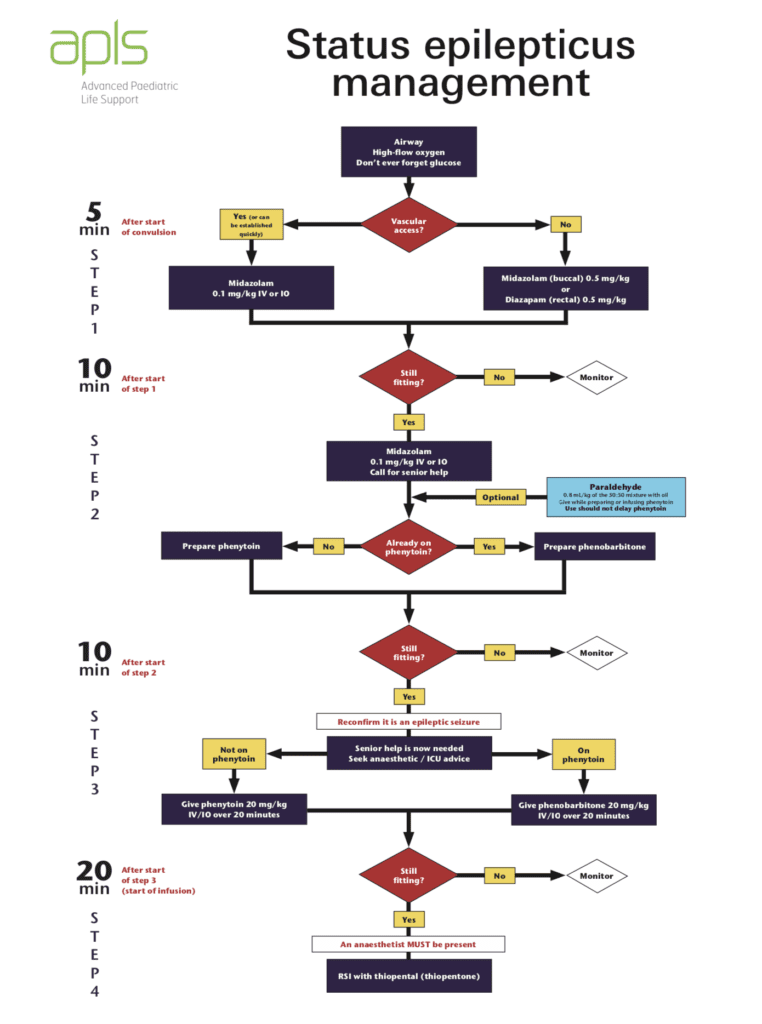
APLS Pediatric Status Epilepticus Management:
EcLiPSE [1]
What They Did:
- Emergency treatment with Levetiracetam or Phenytoin in convulsive Status Epilepticus in children (EcLiPSE)
- Multicenter, open-label randomized clinical trial performed in the UK
- Patients randomized to Levetiracetam (40mg/kg over 5 min – MAX Dose 2.5g) vs Phenytoin (20mg/kg over 20 min – MAX Dose 2g)
Outcomes:
-
Primary: Time from randomization to cessation of convulsive status epilepticus
- Time from randomization: After administration of the final first-line treatment to allow sufficient time to prepare and administer the allocated second-line treatment
-
Secondary:
- Need for further anticonvulsants to manage convulsive status epilepticus after administration of the trial treatment
- Need for RSI because of ongoing convulsive status epilepticus
- Need for admission to critical care
- Serious adverse reactions (including death, Stevens-Johnson syndrome, rash, airway complications, cardiovascular instability, extravasation injury, and extreme agitation)
Inclusion:
- Aged 6mo to <18 years
- Convulsive status epilepticus requiring second-line treatment
Exclusion:
- Absence of myoclonic or non-convulsive status epilepticus
- Known or suspected to be pregnant
- Contraindication or allergy to levetiracetam or phenytoin
- Established renal failure
- Had received a second line anticonvulsant during the presenting episode of convulsive status epilepticus before screening
- Already enrolled in EcLiPSE trial
- Did not require second-line treatment
Results:
-
404 patients randomized
- 93 patients did not require second-line treatment
-
286 patients randomized and treated
- Levetiracetam group: 152 pts
- Phenytoin group: 134 pts
-
Termination of Convulsive Status Epilepticus:
- Levetiracetam group: 70%
- Phenytoin group: 64%
-
Median Time from Randomization to Start of Infusion:
- Levetiracetam group: 11 min (Range 8 – 15min)
- Phenytoin group: 12 min (Range 8 – 17min)
-
Median Time from Randomization to Cessation of Convulsive Status Epilepticus:
- Levetiracetam group: 35min
- Phenytoin group: 45 min
- HR 1.20; 95% CI 0.91 – 1.60; p = 0.20
-
Any Adverse Event:
- Levetiracetam group: 20 events in 16pts (12%)
- Phenytoin group: 23 events in 18pts (14%)
-
Most common adverse event was agitation:
- Levetiracetam group: 11pts (8%)
- Phenytoin group: 4pts (3%)
Strengths:
- Multicenter, randomized clinical trial
- Largest RCT to compare levetiracetam with phenytoin for treatment of pediatric convulsive status epilepticus that was unresponsive to first-line treatment
- Computer generated randomization schedule was produced by an independent statistician who no involvement in the study
- Performed periodic site checks to ensure the correct number of envelopes, that the envelopes were intact, and that sequential numbering system was maintained
- All adverse events were assessed by the principal investigator at each participating site
- Participants were well balanced in baseline characteristics between groups
Limitations:
- Open-label trial as a double-blind design would have been too complex because of the substantially different infusion rates of the two drugs and the life-threatening nature of convulsive status epilepticus
- Cessation of all signs of continuous, rhythmic clonic activity, rather than using fixed timepoints to assess cessation of convulsive status epilepticus was too subjective an outcome
- Although not feasible use of electroencephalogram (EEG) to determine the time of convulsive status epilepticus cessation would have been more precise. Without EEGs, it is not possible to state definitively whether any patients developed non-convulsive status epilepticus
- Timing of randomization meant that convulsive status epilepticus terminated before administration of trial treatment in many cases
- This trial was not powered to show a difference in the number of serious adverse reactions between groups
Discussion:
- It is well known that the longer the duration of convulsive status epilepticus, the more difficult it can be to terminate the seizure and the greater the risk of neurodisability. It seems to me the 35 minute and 45 minute median time to cessation of convulsive status epilepticus is long and makes me question either agent as a second line medication
- Another point of discussion is that Levetiracetam can be administered more rapidly (5 – 10min) than phenytoin (a minimum of 20min) which could potentially terminate convulsive status epilepticus faster with levetiracetam than phenytoin
- An interesting point brought up by the authors is that clinicians may be reluctant to give a loading dose of phenytoin to children in convulsive status epilepticus who are on oral maintenance phenytoin because of potential for cardiovascular toxicity, but there appears to be no similar concerns for levetiracetam
- Ease of drug preparation and administration is also a factor in the management of convulsive status epilepticus. In the EcLiPSE trial, clinical teams reported that it was easier to prepare and administer levetiracetam than phenytoin because of calculations that needed to be performed in reconstituting phenytoin, the number of vials required with phenytoin, and the procedures needed for its administration
- Levetiracetam is also safer, with an even lower incidence of hypotension and respiratory depression compared to phenytoin
Author Conclusion: “Although levetiracetam was not significantly superior to phenytoin, the results, together with previously reported safety profiles and comparative ease of administration of levetiracetam, suggest it could be an appropriate alternative to phenytoin as the first-choice, second-line anticonvulsant in the treatment of paediatric convulsive status epilepticus.”
ConSEPT [2]
What They Did:
- Convulsive Status Epilepticus Paediatric (ConSEPT) Trial
- Open-label, multicenter, randomized controlled trial performed in Australia and New Zealand
- Patient randomized to phenytoin 20mg/kg over 20min vs 40mg/kg levetiracetam over 5min
Outcomes:
-
Primary: Clinical cessation of seizure activity 5 min after the completion of infusion of the study drug
- Because the trial drugs had different optimal infusion rates, this was 10min after starting levetiracetam and 25min after starting phenytoin
-
Secondary:
- Clinical cessation of seizure activity 2hr after the commencement of trial infusions without the need for further seizure management after the initial study drug infusion
- Clinical cessation of seizure activity 2hr after the commencement of trial drug without the need for RSI or further seizure management
- Time to clinical seizure cessation
- Need for RSI for seizure management
- ICU admission
- Serious adverse events including death, serious unexpected airway complications (endotracheal tube, LMA, or cric) in the first 24hr, cardiovascular instability (cardiac arrest or arrhythmia requiring cardiac defibrillation), or other life-threatening events
- Length of hospital and ICU stay
- Seizure status 1 month after discharge or 2 months after randomization (whichever was earliest)
-
Safety:
- Death
- Manual airway repositioning
- Oral or nasal airway placement
- Positive pressure ventilation
- Tracheal intubation
- Fluid bolus
- Cardiac chest compressions
- Cardiac defibrillation
- Allergic reaction
- Extravasation of IV or IO infusions
- Purple glove syndrome
Inclusion:
- Children 3 months – 16 years of age
- Convulsive status epilepticus that failed first-line benzodiazepine treatment
- Received two doses of benzodiazepines (given by parents, paramedics, or hospital staff)
Exclusion:
- Previously enrolled and randomly assigned in the study
- Patients on regular phenytoin or levetiracetam
- Administered second-line anticonvulsants (phenytoin, levetiracetam, phenobarbitone, or paraldehyde) in the past 24hrs
- Management plan stating that patient refractory to phenytoin
- Known contraindication or allergy to phenytoin or levetiracetam
- Convulsive status epilepticus due to an obvious major head injury
- Convulsive status epilepticus due to eclampsia in late pregnancy
Results:
-
233 children
- Phenytoin group: 114
- Levetiracetam group: 119
- Median length of seizure activity before infusion of the first study drug was 73 min (Range 52 – 99)
- Median infusion time for the 1ststudy drug was 21 min (Range 20 – 24) in the phenytoin group and 5 min (Range 5 – 6) in the levetiracetam group
-
Clinical Cessation of Seizure Activity 5min After Completion of Infusion of Study Drug:
- Phenytoin group: 60%
- Levetiracetam group: 50%
- 95% CI 021.0 – 3.5; p = 0.16
-
Clinical Cessation of Seizure Activity at 2hr Without Further Seizure Management:
- Phenytoin group: 54%
- Levetiracetam group: 51%
- 95% CI: -15.9 – 9.7; p = 0.63
-
Median Time to Seizure Cessation (min):
- Phenytoin group: 22 (Range 9 – 49)
- Levetiracetam group: 17 (Range 5 – 30)
- 95% CI013.5 – 3.5; p = 0.25
- No serious adverse events secondary to medications
Strengths:
- Multicenter RCT answering a clinically important question
- Computer generated randomization schedule was produced by an independent statistician who no involvement in the study
- Primary outcome was assessed via video recording to ensure no observer bias due to unblinded nature of the study
- Groups were well balanced in terms of baseline characteristics
- The primary endpoint used in this study was a pragmatic, real-world endpoint based on international consensus recommendations to terminate seizures as soon as possible
Limitations:
- Physicians assessing the primary outcome were not masked to the assigned intervention, and therefore there is potential for bias
- Study designed as a superiority trial on the basis of published reports of efficacy for both drugs at the time of design. Failure to find a difference does not mean that levetiracetam I s statistically equivalent to phenytoin
- Seizure cessation was not confirmed by EEG. Some pseudo-seizures or seizure mimics could therefore have been included
- Timing of primary outcome assessment differed between the two study groups (i.e. 10min vs 25min after the start of study infusions). The longer infusion time for phenytoin could have allowed for lessened effect of benzodiazepines
- Excluded patients who were taking regular levetiracetam or phenytoin, and those with a management plan stating they were refractory to phenytoin (no patient had a management plan stating that they were refractory to levetiracetam). Therefore, the results of this study cannot be extrapolated to this patient population
Discussion:
- Authors state that benzodiazepines alone are effective in terminating convulsive status epilepticus in only around 40 – 60% of cases
- Both Levetiracetam and Phenytoin (given as a single infusion) were effective in controlling seizure activity in 50 – 60% of cases after failure of at least 2 doses of benzodiazepines.
- Only 3 pts (3%) in the phenytoin group and 1 pt (1%) in the levetiracetam group had extravasation of study infusions. All extravasations were promptly identified, and no patient required additional interventions, apart from further IV access
- No patients in either treatment group experienced an arrhythmia requiring intervention in this study
-
Potential cardiac toxicity limits the infusion rate of phenytoin (1mg/kg/min)
- Fosphenytoin is prodrug that is converted too phenytoin…can be given faster than phenytoin (water soluble & does not have propylene glycol as its diluent) and has fewer side effects (i.e. CV arrhythmias, hypotension, and skin necrosis)
- Use of an alternative study drug when the first treatment failed resulted in seizure control without the requirement for further intervention in 27 patients who received phenytoin first (64% of those receiving phenytoin then levetiracetam) and 25 who received levetiracetam first (52% of those receiving levetiracetam then phenytoin)
Author Conclusion: “Levetiracetam is not superior to phenytoin for second-line management of paediatric convulsive status epilepticus.”
Clinical Take Home Points:
- Though both EcLiPSE & ConSEPT hoped to demonstrate that levetiracetam was superior as a second-line agent, neither study demonstrated superiority in efficacy of seizure cessation, nor safety.
-
Both EcLiPSE & ConSEPT compared phenytoin to levetiracetam, however in the US we predominantly use fosphenytoin due to a slightly better safety profile. Admittedly both phenytoin and fosphenytoin are known to cause hemodynamic instability (hypotension, cardiovascular collapse), Toxic Epidermal Necrolysis/Stevens-Johnson syndrome, hepatotoxicity, blood dyscrasias as some of the most severe reactions. However phenytoin is known to have a higher incidence of cardiac arrhythmias (V-fib and AV conduction delays) as well as tissue necrosis with local extravasation. Because of these reasons, the US switched from phenytoin to fosphenytoin as second line antiepileptic agent about 15 years ago. Despite the better safety profile however, both fosphenytoin and phenytoin infusion rates continues to be a limiting factor. Faster infusion rates can increase risk of some of these side effects, hence infusion rate continues to be a limiting issue for both of these medications compared to Levetiracetam.
- Fosphenytoin is prodrug that is converted to phenytoin…can be given faster than phenytoin (water soluble & does not have propylene glycol as its diluent) and has fewer side effects (i.e. CV arrhythmias, hypotension, and skin necrosis)…Levetiracetam is safer, with even lower incidence of hypotension and respiratory depression
-
Given these studies showed equivocal efficacy for seizure cessation, the decision of which drug to use as a second-line agent remains multifactorial:
- Home medications: if on phenytoin at home, giving an additional dose of phenytoin could increase risk of toxicity, thus most providers would avoid double-dosing. Whereas levetiracetam does not appear to have this additive toxic profile. Most providers who note that a patient uses levetiracetam are apt to use this again as a second-line agent.
- Ease of use: the practical aspect of drug preparation and administration is also a factor in the management of convulsive status epilepticus. In the EcLiPSE trial, clinical teams reported that it was easier to prepare and administer levetiracetam than phenytoin because of calculations that needed to be performed in reconstituting phenytoin, the number of vials required with phenytoin, and the procedures needed for its administration.
- Concomitant Psychiatric disorder: patients with known psychiatric disorders should generally avoid levetiracetam. This is not always known upfront in the ED setting, making recommendations for use of this drug in all patients impractical.
- One take-away for us, is that levetiracetam has shifted gears from previously being considered a “prophylactic agent” to now a therapeutic one in the acute care setting. Though it has yet to prove superiority to phenytoin, the fact that we have another option for second line seizure-cessation is a significant one.
- Given all of the above considerations, the decision of which drug to use as a second-line agent needs to be individualized both to patient and institution.
References:
- Lyttle MD et al. Levetiracetam Versus Phenytoin for Second-Line Treatment of Paediatric Convulsive Status epilepticus (EcLiPSE): A Multicentre, Open-Label, Randomised Trial. LANCET 2019. PMID:
- Dalziel SR et al. Levetiracetam Versus Phenytoin for Second-Line Treatment of convulsive Status Epilepticus in Children (ConSEPT): An Open-Label, Multicentre, Randomised Controlled Trial. LANCET 2019. PMID:
For More Thoughts on This Topic Checkout:
- DFTB: Seizing the Truth?
- First10EM:Levetiracetam versus Phenytoin in Status Epilepticus (ConSEPT and EcLiPSE)
- EMLitofNote:Levetiracetam vs. Phenytoin
- St. Emlyn’s Blog: JC – Enter Sandman – Which Agent as Second Line in Paediatric Status Epilepticus?
- The SGEM: SGEM #265 – Total Eclipse of the Seizure – What a Consept
Post Peer Reviewed By: Mizuho Morrison, DO (Twitter: @mizuhomorrison)
The post REBEL Cast Ep71: 2nd Line Therapy for Pediatric Status Epilepticus – EcLiPSE & ConSEPT appeared first on REBEL EM - Emergency Medicine Blog.
