Background: There is a shifting paradigm with persuasive evidence favoring a shorter duration of antibiotics for outpatient management of community-acquired pneumonia (CAP) in children. The SAFER and CAP-IT trials found that short-course antibiotic therapy was not inferior to standard duration therapy. The SCOUT-CAP Trial found that short-course antibiotic therapy was superior to standard therapy. The pooled estimates extrapolated from these randomized control trials bolster the evidence favoring short-course antibiotics in children with CAP.
Background: There is a shifting paradigm with persuasive evidence favoring a shorter duration of antibiotics for outpatient management of community-acquired pneumonia (CAP) in children. The SAFER and CAP-IT trials found that short-course antibiotic therapy was not inferior to standard duration therapy. The SCOUT-CAP Trial found that short-course antibiotic therapy was superior to standard therapy. The pooled estimates extrapolated from these randomized control trials bolster the evidence favoring short-course antibiotics in children with CAP.
Paper: Kuitunen I et al. Antibiotic treatment duration for community-acquired pneumonia in outpatient children in high-income countries – a systematic review and meta-analysis. Clin Infect Dis. 2022. PMID: 35579504
Clinical Question: Is short-course antibiotic therapy, for 3–5 days, safe and effective in children with community-acquired pneumonia stable for outpatient management, compared to the current guidelines of 7–10 days?
What They Did:
- Investigators performed a medical literature search of Pubmed (MEDLINE), Scopus, and Web of Science databases.
- The search was restricted to the last 20 years.
- Two authors individually screened abstracts, and a third author resolved conflicts.
- Researchers assessed the risk of bias with the Cochrane risk bias 2.0 tool.
- Assessed reporting quality using the Grading of Recommendations Assessment, Development, and Evaluation (GRADE) method.
- Reported systematic review and meta-analysis according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA).
Population:
Inclusion Criteria: RCTs focusing on antibiotic treatment duration in CAP among children at age ≥6 months treated as outpatients, regardless of blinding.
Exclusion Criteria:
- Nonrandomized trials and observational studies.
- Studies conducted in middle- and low-income countries
- Different definitions of pneumonia
- Different healthcare facilities and organizations in these countries
- Studies focusing on atypical pathogens, such as M. pneumoniae and Chlamydia pneumoniae.
- Studies focusing on pneumonia treatment duration in children aged <6 months.
- In the included studies, patients with complicated pneumonia were excluded (i.e. requiring inpatient treatment)
Intervention:
-
Shorter antibiotic treatment duration (3–5 days).
Comparator:
-
Standard antibiotic treatment duration (7–10 days).
Outcomes:
Primary Outcomes:
- Need for antibiotic retreatment within 1 month of starting the initial treatment.
- Hospitalization
- Treatment failure (Composite of need for antibiotic retreatment and hospitalization)
Secondary Outcome: Antibiotic-related adverse effects
Results:
- 779 records retrieved
- 238 duplicate records removed
- Assessed 541 abstracts
- 530 studies excluded
- 11 full texts evaluated
- 4 studies included in the final analysis
- Mean age of children between 28 and 36.8 months
- 3 of the 4 trials were non-inferiority trials and only one was a superiority trial
Critical Results:
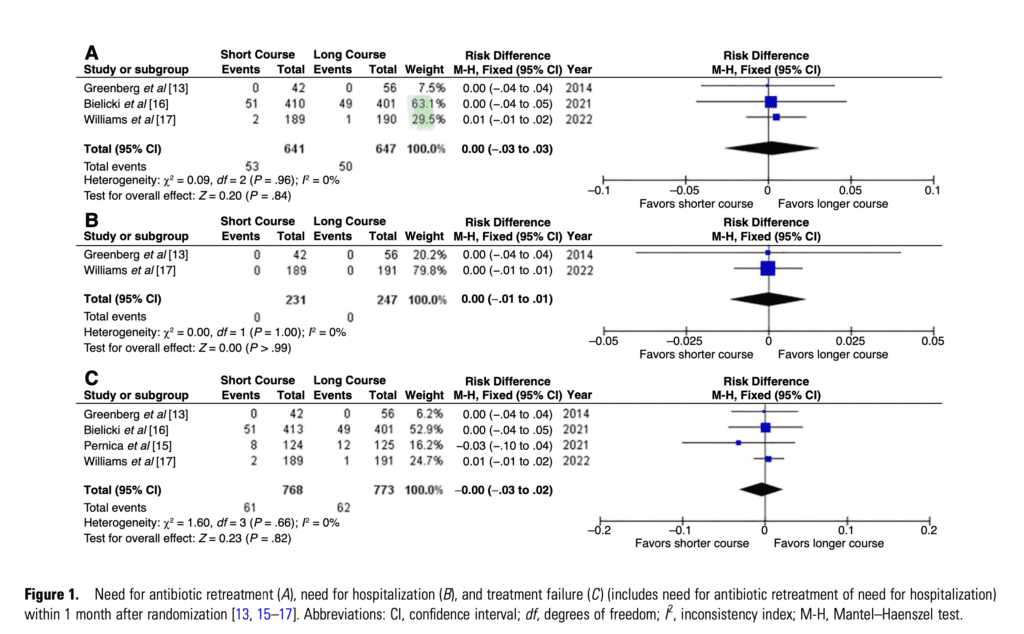
- Need for Antibiotic Re-treatment
- 3 of 4 studies with 1288 children included.
- 8.3% in the short-course and 7.7% in the long-course group.
- (RD, 0.00 [95% CI .03 to .03]; I2 = 0%)
- The quality of evidence was high, and the risk of bias was low.
-
Need for Hospitalization
- 2 of 4 studies with 478 children included
- No hospitalizations occurred in either of the studies
- (RD, 0.00 [95% CI .01 to .01]; I2 = 0%)
- The quality of evidence was high, and the risk of bias was low.
-
Treatment Failure
- 4 of 4 studies with 1541 children included.
- 7.9% in the short-course and 8.0% in the long-course group
- (RD, 0.00 [95% CI, .03 to .02]; I2 = 0%)
- The quality of the evidence was high, and the risk of bias was low.
- Adverse Events Related to Antibiotic Treatment
- 2 of 4 studies 1194 children included.
- (RD, 0.00 [CI .05 to .05]; I2 =0%)
- The quality of the evidence was moderate, and the risk of bias was assessed as being of some concern.
Strength:
- Investigators asked a narrowly focused, patient-centered clinical question.
- Performed a comprehensive search of multiple databases.
- Included only RCTs.
- Applied rigorous methodology in conducting the review and used PRISMA guidelines.
- Explicitly described the risk of bias in each study using the Cochrane Bias assessment tool—the risk of bias was low in all but one trial.
- Addressed quality with the GRADE method—quality of evidence was high in 3 of 4 trials and moderate in the 4th.
- Heterogeneity was low, and results were similar across all trials.
- Three studies exclusively used high-dose amoxicillin (80–100 mg/kg/d).
- The remaining study used both low-dose (35–50 mg/kg/d) and high-dose amoxicillin (80–100 mg/kg/d).
- Appropriate use of noninferiority design in three included noninferiority studies.
- The noninferiority threshold was conservative and less than 10% in all three noninferiority trials.
- Explicitly described and explained differences among the included trials.
- Presented data that appears to be ready for clinical application.
Limitations:
- Did not screen bibliographies for additional articles.
- Did not search ClinicalTrials.gov for unpublished trials.
- Excluded trials from low-income countries—It’s unclear what determines a low-income country.
- Original trials excluded children with many chronic medical conditions.
- The definition of pneumonia varied between the studies (i.e. some of these may have been viral infections)
- Chest x-ray was required in only two studies.
- Only one trial tested patients for respiratory viruses, and 83.9% (short-course group) and 80.1% (long-course group) tested positive for viruses. Would these kids have gotten better either way without antibiotics?
- It’s unclear how patients might respond to short-course therapy with other medications and dosing regimes.
- Most included patients came from two trials, and more than half came from one.
- The original purpose of the meta-analysis was to compare clinical cure rates between short-course and long-course arms, but the clinical parameters expressing cure were heterogeneous between trials and could not be pooled to produce any summary estimates.
- Low failure rates in the included studies resulted in using RDs instead of risk ratios.
Discussion:
Inside the Numbers:
-
No additional children required antibiotic retreatment when treated with short-course antibiotic therapy compared to standard treatment duration.
(RD, 0.00 [95% CI .03 to .03]; I2 = 0%). -
No additional children required hospitalization when treated with short-course antibiotic therapy compared to standard treatment duration.
(RD, 0.00 [95% CI .01 to .01]; I2 = 0%). -
No additional children had a treatment failure when treated with short-course antibiotic therapy compared to standard treatment duration.
(RD, 0.00 [95% CI, .03 to .02]; I2 = 0%). -
No additional children had antibiotic-related adverse events when treated with short-course antibiotic therapy compared to standard treatment duration.
(RD, 0.00 [CI .05 to .05]; I2 =0%). - If a longer course of antibiotic therapy does not confer any advantage or decrease the risk of outcomes, parents would likely choose the shorter duration of therapy. Surprisingly, there was no difference in adverse events with longer antibiotic duration. We might assume that longer exposure to medicines would cause more side effects.
Diagnosing Pneumonia:
Diagnosing bacterial pneumonia in children remains elusive. Many children with abnormal chest X-rays will have a viral etiology, and some children with a positive viral panel may have coinfection with a bacterial pathogen. Only 2 of 4 trials required a chest X-ray to diagnose pneumonia, and only 1 of 4 trials required viral testing. While pragmatic, in a randomized control trial, homogeneity in the enrolled population creates more certainty in the validity of the results. Additionally, the symptoms of CAP are similar to URI. Chest X-ray is by no means a perfect diagnostic test, but an abnormal chest x-ray adds certainty that the population being studied, at minimum, has pneumonia. It’s possible some patients with URIs were misdiagnosed as having CAP. These patients would likely improve regardless of treatment length.
Author’s Conclusions: “Short treatment was equally safe and effective as longer treatment of 7–10 days for CAP in outpatient children aged ≥ 6 months in high-income countries. We suggest that this shorter treatment for 3–5 days can now be implemented into clinical practice.”
Our Conclusion:
We agree with the author’s conclusion. This meta-analysis and four original RCTs provide compelling evidence to recommend using short-antibiotic therapy for children with CAP stable for outpatient treatment. Diagnosing bacterial pneumonia in the pediatric population remains problematic, but clinicians should prescribe the shortest duration of antibiotic therapy amenable to cure. Based on the constellation of data available, 3–5 days of antibiotics is perhaps the best option.
Clinical Bottom Line:
Prescribe 3–5 days of antibiotic therapy In children ≥6 months of age with CAP from high-income countries who are stable for outpatient management.
For More Thoughts on This Topic, Checkout:
- Don’t Forget the Bubbles: How long should we treat children with pneumonia for?- the results of CAP-IT,
- REBEL EM: The SAFER Trial: Pediatric CAP-Amoxicillin 5 days vs 10 days
- REBEL EM: The CAP-IT Trial: Amoxicillin Dose and Duration in Children with Community-Acquired Pneumonia
- REBEL EM: The SCOUT–CAP Trial: 5d Abx vs. 10d Abx in Pediatric CAP
- The SGEM: Meet Me Halfway on the Duration of Antibiotics for Non-severe Pediatric CAP
- The SGEM: SGEM#338 – Are Children With CAP Safe and Sound if Treated for 5D Rather Than 10D of Antibiotics?
References:
- Pernica JM et al. Short-Course Antimicrobial Therapy for Pediatric Community-Acquired Pneumonia: The SAFER Randomized Clinical Trial. JAMA Pediatr 2021. PMID: 33683325
- Bielicki J et al. Effect of Amoxicillin Dose and Treatment Duration on the Need for Antibiotic Re-treatment in Children With Community-Acquired Pneumonia: The CAP-IT Randomized Clinical Trial. JAMA. 2021. PMID: 34726708
- Williams DJ et al. Short- vs. Standard-Course Outpatient Antibiotic Therapy for Community-Acquired Pneumonia in Children: The SCOUT-CAP Randomized Clinical Trial. JAMA Pediatr. 2022;e215547. PMID: 35040920
Post-Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post Short Course Antibiotics for Peds CAP: A Systematic Review and Meta-Analysis appeared first on REBEL EM - Emergency Medicine Blog.