
 “I’ve been in this game for years, it made me an animal
“I’ve been in this game for years, it made me an animal
There’s rules to this sh*t; I wrote me a manual”
— Notorious BIG
You know, whether you sling crack rocks or not, there are some sage words of wisdom in the late Notorious BIG’s Ten Crack Commandments. Life pearls like “Never let ‘em know your next move” and “Never keep no weight on you” have helped guide me through some challenging life decisions.
1. Never let no one know how much dough you hold
2. Never let ‘em know your next move
3. Never trust nobody
4. Never get high on your own supply
5. Never sell no crack where you rest at
6. That Goddamn credit, forget it
7. Keep your family and business completely separated
8. Never keep no weight on you
9. If you ain’t getting bags stay the f*ck from police
10. A strong word called consignment; If you ain’t got the clientele say hell no
Table 1. The ten crack commandments.
While not much is new in the world of hustlin’, when it comes to trauma resuscitation, the game done changed*. It was easier in the old days: 2L of crystalloid for a hypotensive patient, and then blood. While new science on trauma resuscitation has helped us understand how flawed that paradigm is, the new school can be some tricky water to navigate. From damage control to fibrinogen, from TXA to thromboelastometry, there is no doubt that resuscitating a bleeding trauma patient is a more nuanced endeavor than we originally envisioned it.
So, inspired by Biggie’s Descartesian ten-point discourse on method, I present the ten rules of the contemporary trauma resuscitation game as I see them – backed by science, and occasionally editorialized with personal opinion.
* Some academics have argued that the game has in fact not changed, but has just become more fierce; see Slim Charles vs. Cuddy
1. Preventing death from hemorrhage is a team sport, and resuscitationist’s game
If you’re going to die from injury, statistically speaking it will be due to a severe traumatic brain injury; close behind that is death from exsanguination. And while much of the damage from head injury is spoken for at the time of accident, the initial resuscitation of a bleeding trauma patient can have a tremendous impact on survival. To be clear, the most important step in managing these patients is surgical source control – most patients with massive hemorrhage need an operation to stay the hemorrhage. The state in which a patient arrives to the operating room or the intensive care unit – alive or near death, cold and coagulopathic or warm and well perfused – is up to you.
2. If you anticipate the need for more than 3-4 units of packed cells in the first few hours, you’re in it
The young patient with a thoraco-abdominal gunshot wound who presents hypotensive and tachycardic is exactly the type of patient who will benefit from the principles of damage control resuscitation – hemostatic blood product administration, restrictive fluid administration, permissive hypotension, early surgical control for blood loss. For others, it can be a bit trickier. As it turns out, physician gestalt is probably not so great for predicting the need for massive transfusion and calculated parameters like the shock index and the ABC score don’t seem to fare much better (Injury 2015; 46(5): 807-813).
Decision-making in these circumstances is likely dependent on two things: a good sense of situation awareness, and the ability to rapidly assess response to your initial resuscitative efforts. Synthesizing these concepts, a patient who is likely to need in excess of 3-4 units of red cells during the first several hours of resuscitation is likely to benefit from a damage control strategy (BMJ 2012; 345: 38-42). This should be your practical trigger for initiating ratio-based resuscitation (part 4), initiating a massive transfusion protocol (part 6) and mobilizing the necessary operative resources.
3. One in four trauma patients is bleeding abnormally from the get-go – and here’s why
Perhaps as important as what trauma patients bleed is how they bleed – about 25% of severely injured trauma seem to bleed abnormally. The phenomenon of an early coagulopathy in trauma – which goes by many names, including the Acute Coagulopathy of Trauma-Shock (ACoTS) – can occur soon after injury, and is physiologically distinct from the DIC-like phenomenon associated with the “lethal triad” (Figure 1). While ACoTS is probably a heterogenous entity, it seems to have a lot to do with early hyper-fibrinolysis and factor consumption, rather than the late, dilutional coagulopathy associated with excessive crystalloid administration.
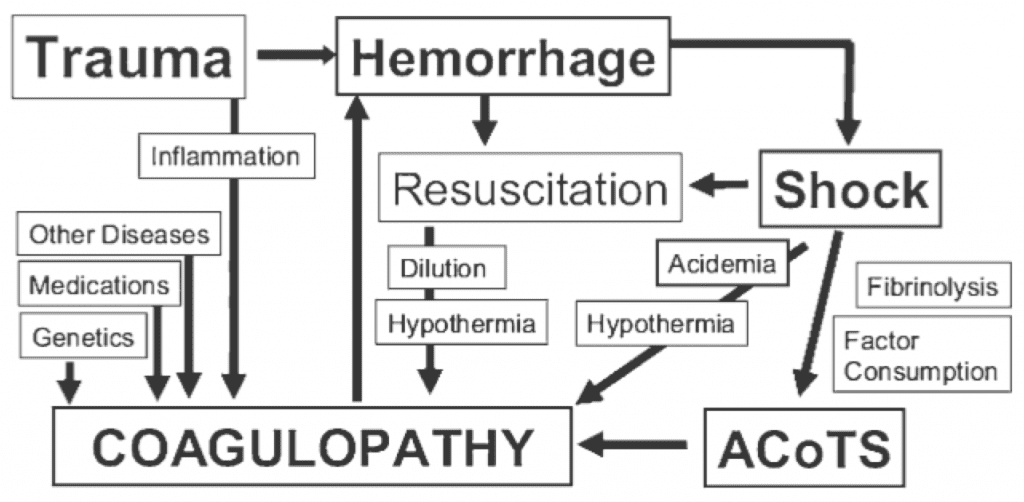
Figure 1. The Acute Coagulopathy of Trauma-Shock (ACoTS) is an early phenomenon, occurring in about 25% of severely injured patients. From: Chest 2010; 137: 209-220
Predicting which patients will have an early coagulopathy is a challenge, and entities hyper-fibrinolysis are largely invisible on standard laboratory coagulation testing. Your mental posture with a severely injured trauma patient should be that an early coagulopathy exists from the get-go, and manage accordingly.
4. Limit early fluid administration in bleeding patients
Excessive crystalloid administration is associated with hypothermia, coagulopathy and death in bleeding patients. A strategy of permissive hypotension is likely beneficial in the early stages of resuscitation, particularly for penetrating trauma – in the pre-hospital setting and during the early stages of resuscitation, it is reasonable to restrict fluid boluses to patients who lose their radial pulse or are demonstrating signs of abnormal end-organ perfusion.
Even ye olde 9th edition of ATLS is hip to this, changing its initial fluid challenge recommendations from 2L crystalloid to 1L, giving consideration to the early use of blood products. I would further editorialize to suggest that if you think your patient is bleeding and you have ready access to blood products, you can skip crystalloid all together and go straight for the good stuff.
A few additional points to ponder:
- Colloids vs. crystalloids: the most recent Cochrane review on this failed to show a mortality benefit of colloids over crystalloids (Cochrane Database Syst Rev 2001, CD000567)
- Patients with a severe head (GCS < 8) and spinal injury are probably an exception to the permissive hypotension strategy; in such cases, a mean arterial pressure of > 80 mmHg has been recommended to promote cerebral perfusion (Critical Care 2013, 17: R76)
- Hypertonic saline solutions are a safe and reasonable option for the hypotensive, head injured patient, although there is no conclusive evidence of their benefit (Cochrane Database Syst Rev 2001, CD000567)
5. Give blood products (red cells, plasma, platelets) in a balanced ratio that mimics that of whole blood
Trauma patients don’t just bleed red blood cells. They lose plasma, platelets and clotting factors, too. There are three ways to try and provide balanced blood product administration: by following lab values, by transfusing blood products in set ratios, or by following point-of-care coagulation testing like rotational thromboelastometry (ROTEM).
Lab-based: This is the old school. Resuscitate, follow a CBC and an INR, and give blood products according to the results. While it is still essential to measure and follow these values as part of your ongoing resuscitation, the main criticism of this approach is that it is reactive rather than proactive – responding to a coagulopathy once it has developed is probably too late. Furthermore, the hyper-fibrinolysis that typifies ACoTS is largely invisible to standard lab testing, making it difficult to treat what you can’t measure.
Ratio-based: There is no question that concept of balanced blood product administration based on set ratios has become the dominant paradigm in modern trauma resuscitation. The idea stems from research in battlefield medicine, where it seemed (albeit retrospectively) that transfusing an equal ratio of plasma to red blood cells was associated with improved survival (Figure 2).
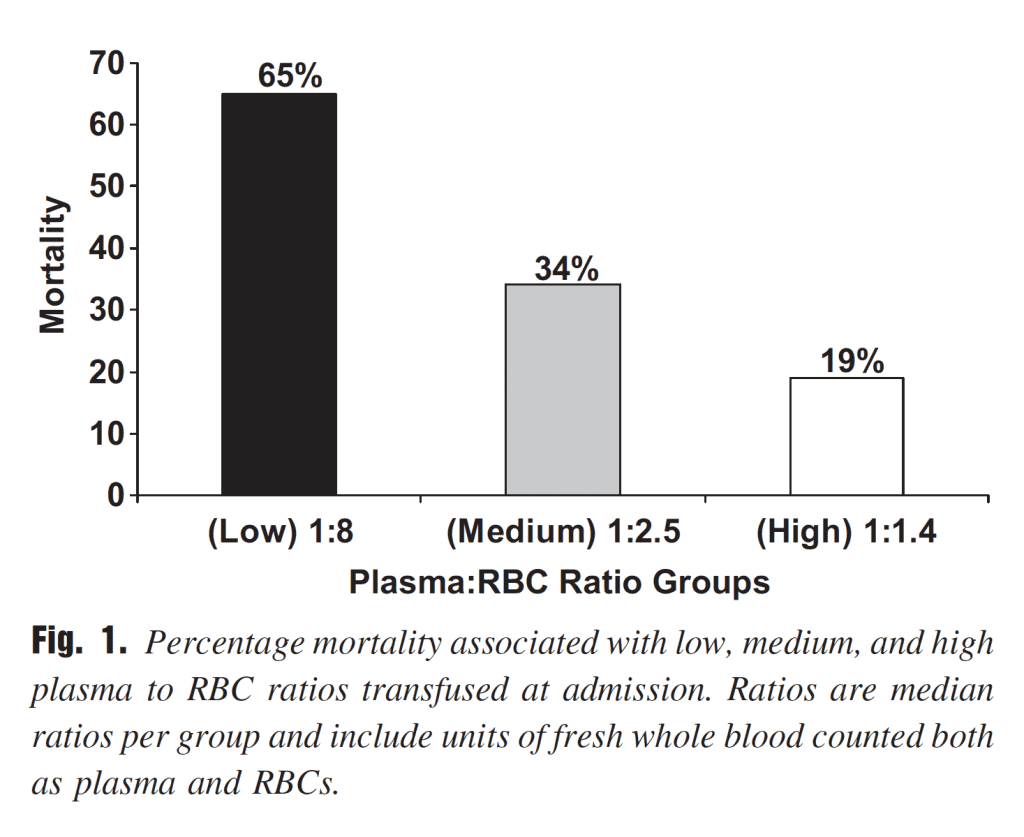
Figure 2. Early observations from battlefield resuscitation, albeit retrospective, seemed to favour a 1:1 ratio of plasma to red blood cells. From: J Trauma 2007; 63:805-813
Balanced blood product administration and identifying early coagulopathy are key elements of the damage control approach (Figure 3).
- Balanced administration of blood products
- Prevention/reversal of coagulopathy
- Minimize crystalloid administration
Figure 3. Principles of damage control resuscitation. From: JAMA 2015, 313(5): 471-482
You would be justified in viewing this with some circumspect. The majority of evidence for raito-based resuscitation comes from retrospective reviews, with all the inherent biases and limitations therein. In 2014, Khan, Brohi et al. published a prospective study looking at the effect of ratio-base resuscitation at either a 1.5:1 or 1:1 (red cells: plasma) on the ability to reverse coagulopathy and clear lactate in massively bleeding patients (J Trauma 2014, 76(3): 561-568). The result – lower ratios were not associated with improved coagulation parameters or adequacy of resuscitation as measured by lactate clearance (giving the paper its fantastic title: “Hemostatic resuscitation is neither hemostatic nor resuscitative”).
Recent, prospective trials like PROMMTT (JAMA Surgery 2013, 148(2): 127-136) and more recently PROPPR (JAMA 2015, 313(5): 471-482) have helped shed some light on the issue of ratio-based resuscitation. Results from PROMMTT suggested a possible survival advantage to 1:1 resuscitation in the first six hours (when hemorrhagic shock was the predominate cause of death) amongst patients who received > 3 units of red cells in the first 24 hours, although over-all survival was not improved. PROPPR was the first randomized trial to examine the effect of 1:1:1 (adding platelets as the third blood product) to vs. 1.5:1:1 – check out Salim’s awesome review . Although there was no difference in over-all mortality at 24 hours and 30 days, death from exsanguination and hemostasis were both improved in the 1:1:1 group. That is to say, if you’re going to die from massive hemorrhage, it’s possible that resuscitation following a 1:1:1 ratio might help you do so less frequently.
If you can’t resuscitate with high ratios of plasma and packed cells (that is, if your access to plasma is limited or restricted), there is at least some evidence that resuscitation with 1L of crystalloid per unit of red cells is associated with improved survival, in the spirit of “resuscitating the interstitum” (J Trauma 2011; 71(2 Suppl 3): S380-383).
ROTEM-based: A full discussion of thromboelastometry (TEG) and it’s close cousin, rotational thromboelastography (ROTEM), is beyond the scope of this post. Proponents of goal-directed resuscitation, like my boss and world ROTEM expert Dr. Sandro Rizoli would argue that ACoTS isn’t one thing, it’s likely a bunch of things, and that it’s different for every patient. In some cases, abnormal bleeding is caused by factor loss, in others hyper-fibrinolysis, in other still a fibrinogen deficiency. ROTEM is a point-of care test that examines blood clotting in real time, providing a series of numeric and graphic parameters that can be used to target resuscitation to the specific problem at hand. A more in-depth review of TEG and ROTEM can be found HERE .
We are currently trialing this for all trauma patients at St. Michael’s hospital, and I’ll admit it looks pretty cool. The problem is, although ROTEM seems to fairly accurately predict the need for massive transfusion, a 2011 Cochrane review suggested there is currently a paucity of evidence on the accuracy of using it to guide treatment decisions (Figure 4, 5).
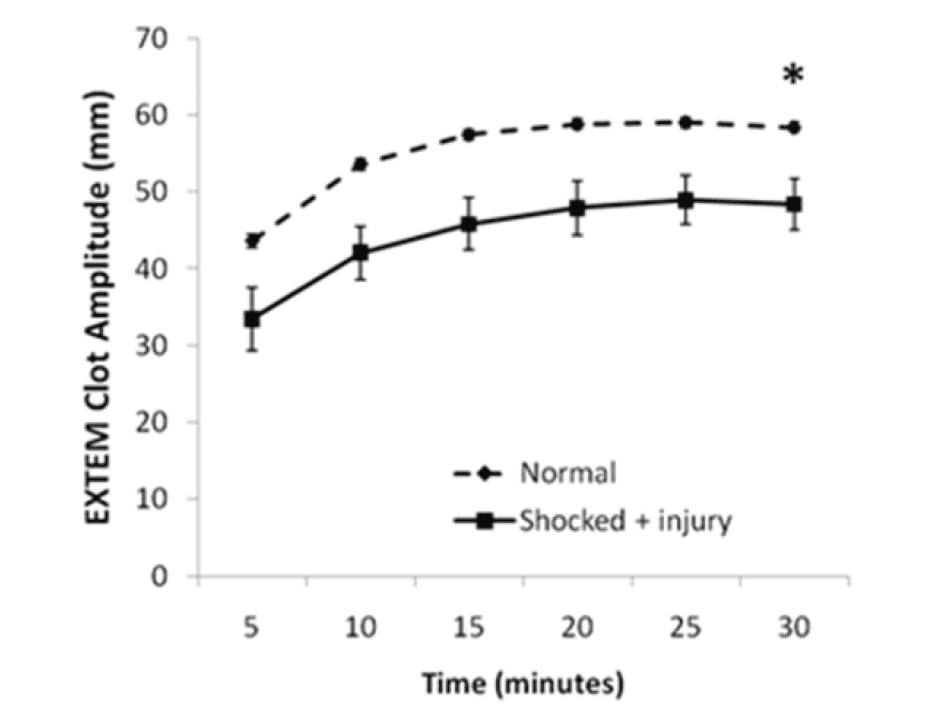
Figure 4. Rotational thromboelastography (ROTEM) seems to predict injury severity and need for massive transfusion. From: Crit Care Med 2011, 39(12): 2652-2658
Figure 5. Walk in to the trauma room at St. Michael’s Hospital, and you’ll se this above the door – a dedicated screen for displaying rotational thromboelastography (ROTEM) values for all trauma patients.
6. Use a massive transfusion protocol to guide blood component therapy
Effective resource utilization is key during complex trauma resuscitation. When it comes to delivering blood products, it’s easy to lose track of units given, ratios, adjuncts, and requirements for ongoing monitoring. Having a massive transfusion protocol (MTP) helps to address these issues by providing organized, timely and predictable delivery of blood products. A well-structured MTP hastens the delivery of blood products, promotes ratio-based resuscitation, decreases waste and improves system flow and communication. Perhaps just as importantly, a functioning MTP frees up cognitive resources for the team leader to focus on other elements of the resuscitation.
A before-after study in 2009 examined the effect of an MTP on mortality in massively transfused trauma patients (Figure 6,J Am Coll Surg 2009; 20(2): 198-205). Surprisingly, the mortality dropped from 45% in the pre-phase to 19% in the post phase, even though the ratio of blood products and the total amount given was comparable in both phases. The authors hypothesize that it was the tool itself that was responsible for the survival bump, by improving system cohesion and communication during complex events.
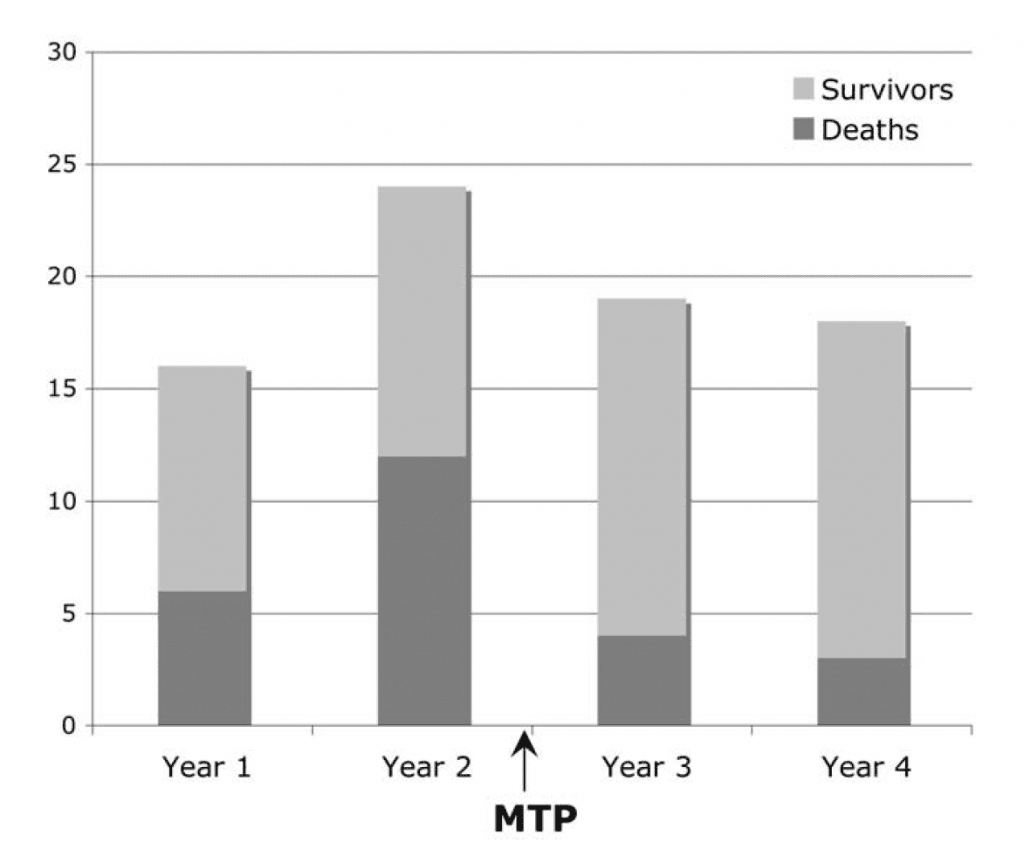
Figure 6. In this before/after study, the use of a massive transfusion protocol (MTP) had a positive influence on mortality in trauma, despite not altering the amount of blood products or ratios given. From: J Am Coll Surg 2009; 20(2): 198-205
If your shop does not have a MTP, it is well worth the institutional effort to create one. Feel free to contact me; happy to share what we’re doing at St. Michael’s.
7. Give a gram of tranexamic acid early to all patients who you think are bleeding
Tranexamic acid (TXA) is an anti-fibrinolytic agent that can/should be used early in the resuscitation of bleeding trauma patients. In CRASH-2 (Lancet 2010; 376: 23-32), 1g of TXA given as an early bolus followed by an infusion of 1g over the ensuing 8 hours was associated with an absolute risk reduction of 1.5%. Not convinced? Here’s a high level summary of the evidence from Karim Brohi himself:
Figure 7. Nothing I can say will add much to these words.
But there’s more to know about TXA than just “you should give it – no really, you should”. The effect of TXA on mortality in bleeding trauma patients is very time-dependent, conferring a huge survival advantage if given early. Like, less than one hour after injury, probably in the pre-hospital setting early. In 2011, a sub-group analysis of the 1 063 deaths due to bleeding from the CRASH-2 database suggested a dose-response effect of timing of administration of TXA and risk of death that favoured very early administration (Figure 8, Lancet 2011, 377: 1096-1011).
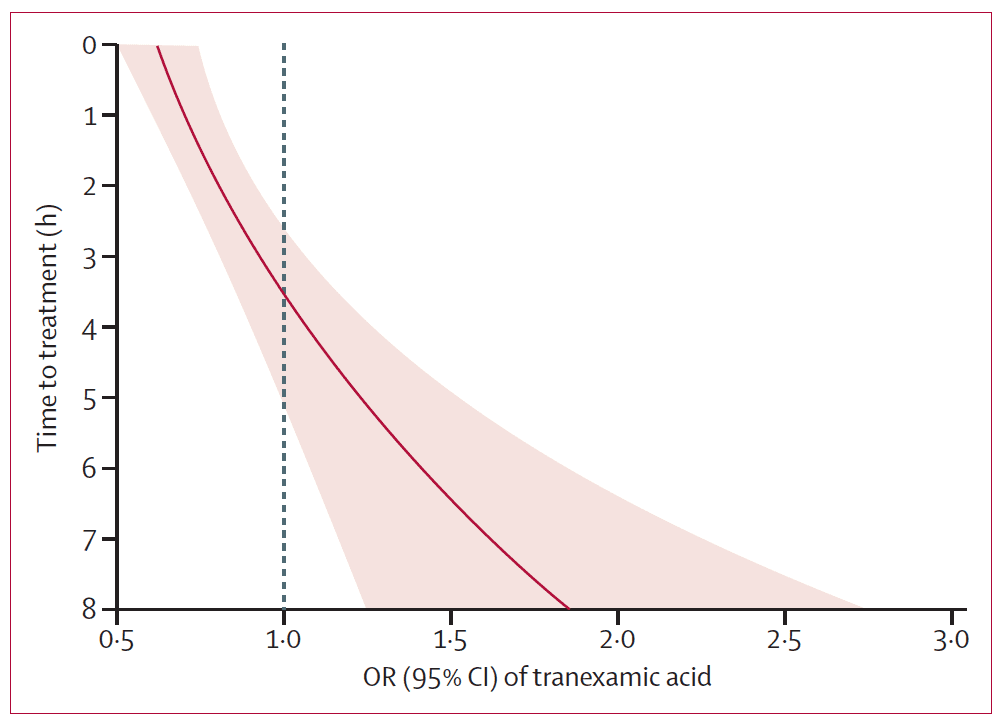
Figure 8. Tranexamic acid has a big impact on mortality if given early ( 3h after injury, although mortality for all comers was not affected. The reasons for this are poorly understood, but hypothesized to be linked to the possible deleterious effects of an anti-firbrinolytic in the late stages of trauma coagulopathy, where a pro-coagulopathic, DIC-like picture predominates.
At our trauma hospital we try to encourage the use of TXA with the following rubrict: If you’re thinking about giving blood, you should also be giving (early) tranexamic acid.
A common (largely Western world) criticism of CRASH-2 was that it was conducted by in large, in non-North American hospitals, where trauma care can look quite different. Whether you accept that as a strength or a weakness of the conclusions (I’d favour the former), you might be reassured by results from the MATTERS study (Figure 9, Arch Surg 2012;147:113-9), which examined the effects of TXA in a modern combat hospital setting much more akin to a trauma centre. The results – patients who received TXA according to the CRASH-2 protocol had a lower unadjusted mortality, despite being more severely injured. The impact was particularly profound in massively transfused patients, with a number needed to treat of seven to prevent death or worsening coagulopathy.
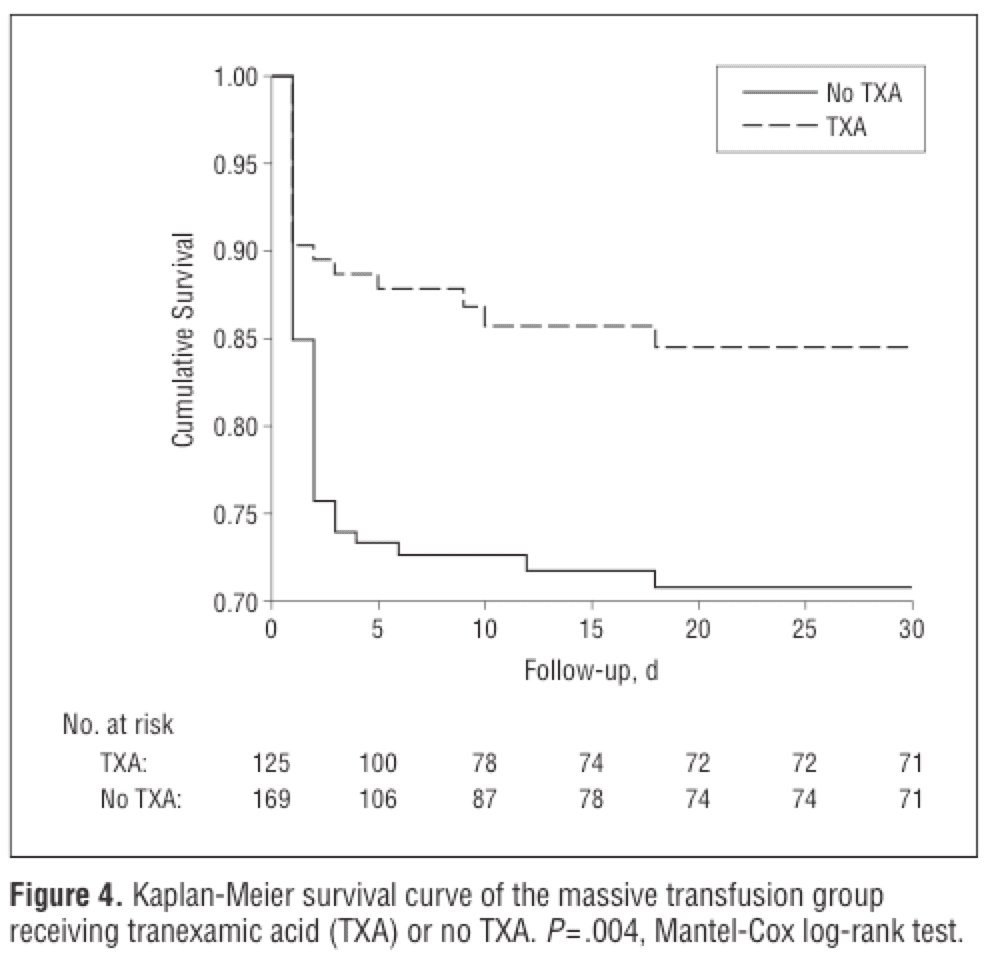
Figure 9. The use of tranexamic acid in massive resuscitation is associated with a number needed to treat of seven to prevent death or coagulopathy. From: Arch Surg 2012;147:113-9
8. A fibrinogen of less than 1.0 g/L is an indication for cryoprecipitate
Cryoprecipitate is the Rodney Dangerfield of blood products – it don’t get no respect. Fibrinogen is the primary substrate for clot formation (along with platelets), present in g/L quantities in whole blood. There is a consistent link between falling fibrinogen and mortality in trauma: In a massively bleeding patient, fibrinogen can drop to clinically important levels faster than other blood components, and its breakdown and synthesis are deleteriously affected by hypothermia and acidosis — leading to a vicious cycle of abnormal clot formation and excessive bleeding (Figure 10).
The key is to measure and follow serum fibrinogen in bleeding patients – a fibrinogen less than 1.0 g/L identifies a hypofibrinogenemic state, the antidote for which is cryoprecipitate.
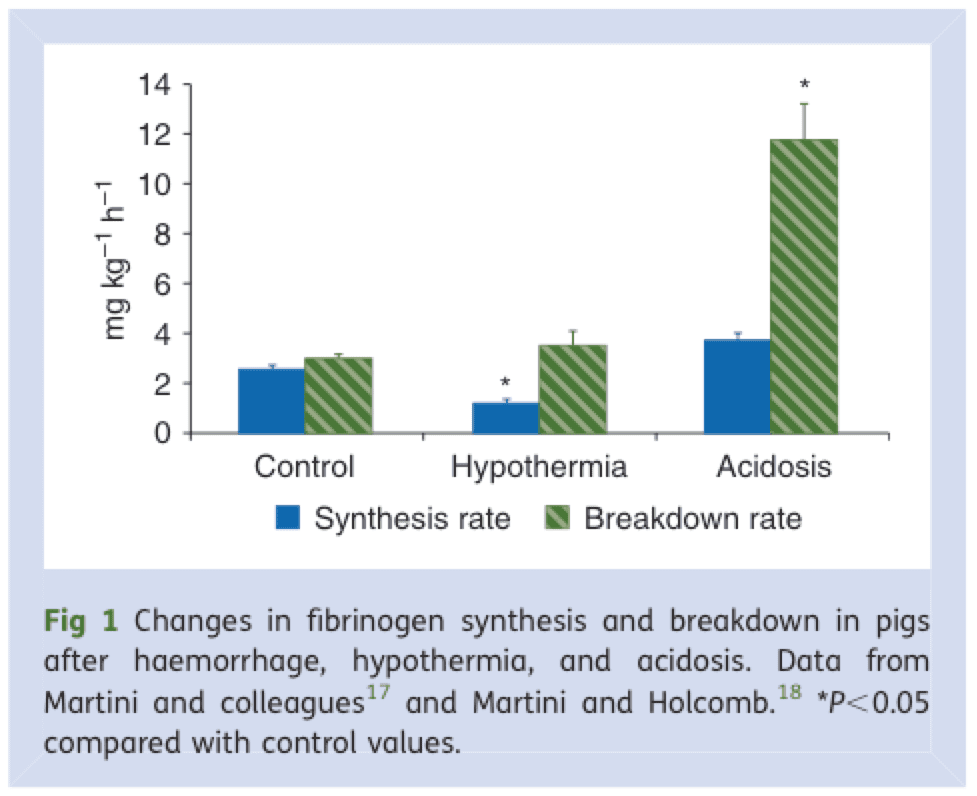
Figure 10. Fibrinogen synthesis and breakdown rate are negatively influenced by hypothermia and acidosis, contributing to a vicious cycle of coagulopathy. From: BJA 2010, 105(2): 116-121
If you’re going to give cryoprecipitate, don’t mess around – the initial starting dose should be at least 10 units (perhaps 8 units for small adult, 12 units for a big one) – some would argue for starting as high as 15 or 20 units. Note as well that the trigger for transfusion cryoprecipitate is subject to some regional variability, with a higher cutoff (1.5 or 2.0 g/L) favored in some areas of Europe and Australia.
9. If you resuscitate based on vital signs alone, you will under-resuscitate about 50% of trauma patients
This is disturbing: a published report from the UK Trauma Audit Research Network from 1989-2007 demonstrated that as patients move towards stage four shock ( > 2L blood loss), the average heart rate increased from 85 to 92, systolic blood pressure was often maintained at > 120 mmHg, and respiratory rate and level of consciousness did not change (Resuscitation 2011;82:556-9). If working off of vital signs alone, about half of trauma patients would be under-resuscitated (J Trauma 2000; 48: 8-13). In fact, only when blood loss approaches half the circulating volume or occurs very rapidly is there a reliable relationship between cardiac output and blood pressure (CCM 1993; 21: 218-23). This is of course assuming that a patient’s physiology isn’t altered by the effects of extremes of age, or medications – which it often is.
10. Use a base deficit, delta lactate and urine output to guide ongoing resuscitation
Whether under or over-resuscitation is the issue, clinical judgment can be augmented with tests that more accurately reflect metabolic derangements at the tissue level. Serum lactate and base are tightly coupled with shock and volume loss. Measured from a venous or arterial blood gas, a base excess of greater than -6 mmol/L (that is, a bigger negative number) is consistently associated with shock, and may provide clues to the presence of “occult shock” in patients whose physiology is otherwise not revealing (Figure 11).
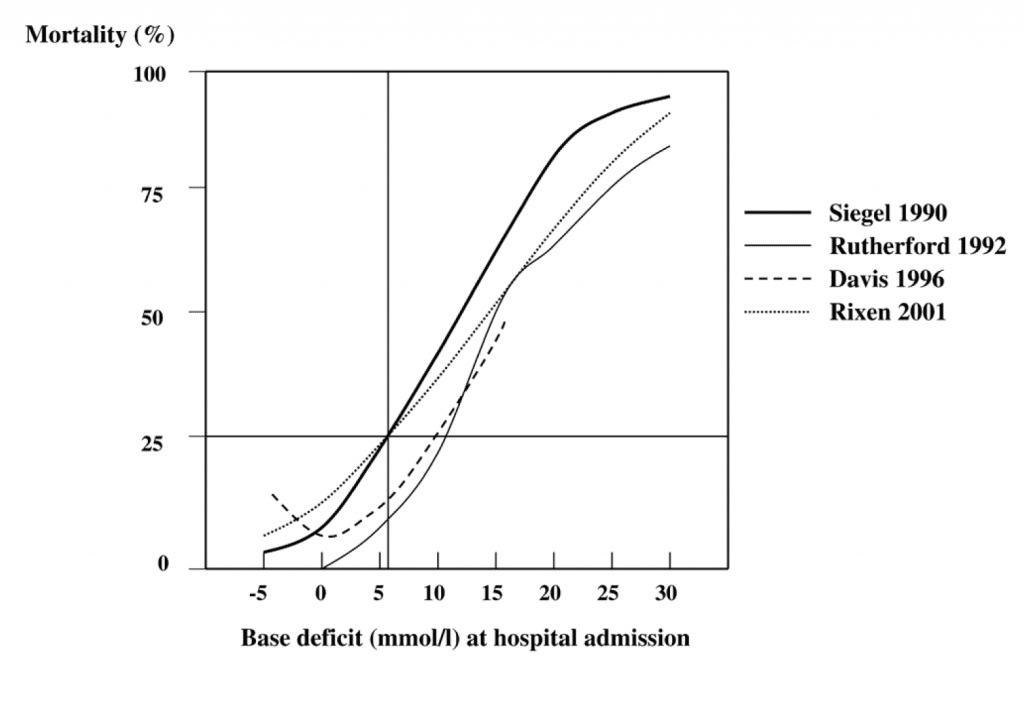
Figure 11. The base deficit and serum lactate give you similar information. A base deficit (the absolute value of the base excess) of 5-6 mmol/L or larger is fairly consistently associated with a shock state; the LD50 for base deficit is somewhere between 11-12 mmol/L. From: Critical Care 2005 9:441
Serum lactate gives you the same information as the base excess. One interesting paper from Anesthesiology (Anesthesiology 2012, 117(6): 1276-88) looked at the association between early (0-2h) lactate clearance and survival in bleeding trauma patients; a 20% per hour drop in serum lactate over the first two hours of resuscitation seems to be a reasonable marker of adequacy of resuscitation, and is associated a lower mortality (Figure 12).
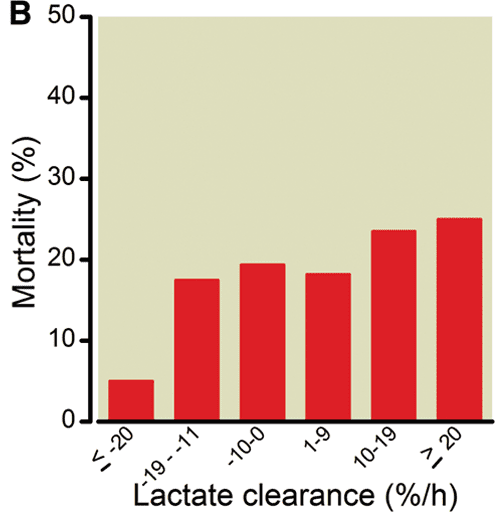
Figure 12. If you’re clearing lactate at a rate of 20% per hour for the first two hours, you’re doing probably doing okay. From: Anesthesiology 2012, 117(6): 1276-88
Finally, don’t forget the foley – in addition to being a useful adjunct for diagnosing genitourinary tract injuries, it’s probably the best in vivo marker of end-organ perfusion during the early stages of trauma resuscitation. If your patient is producing 30-50 cc/h of urine or more per hour, renal perfusion is probably adequate, and so is your resuscitation.
Figure 13. The foley catheter is an essential adjunct during massive resuscitation. If your patient is making urine at a rate of > 50cc/h, your resuscitative efforts are probably adequate.
“Follow these rules, you’ll have mad bread to break up,
If not, twenty-four years on the wake up …”
Post Peer Reviewed By: Salim Rezaie (Twitter: @srrezaie)
The post Ten (Trauma Resuscitation) Commandments appeared first on REBEL EM - Emergency Medicine Blog.
