
 Background: Alteplase has been the accepted thrombolytic for acute ischemic stroke (AIS) for 25 years. However, recent data has emerged regarding tenectaplase as an alternative. Tenecteplase is a modified form of alteplase, with a lower cost and more favorable pharmacokinetic profile allowing for bolus injection. Specifically, it has a higher fibrin specificity and lower affinity to plasminogen activator inhibitor (PAI-1) with a slightly longer half-life.
Background: Alteplase has been the accepted thrombolytic for acute ischemic stroke (AIS) for 25 years. However, recent data has emerged regarding tenectaplase as an alternative. Tenecteplase is a modified form of alteplase, with a lower cost and more favorable pharmacokinetic profile allowing for bolus injection. Specifically, it has a higher fibrin specificity and lower affinity to plasminogen activator inhibitor (PAI-1) with a slightly longer half-life.
Paper: Oliveira M et al. Tenecteplase for Thrombolysis in Stroke Patients: Systematic Review With Meta-Analysis. AJEM 2021. [Link is HERE]
Clinical Question: What is the efficacy/safety of tenecteplase vs alteplase in the treatment of acute ischemic stroke?
What They Did:
- Systematic review and meta-analysis looking at trials comparing alteplase vs tenecteplase
- Searched PubMed and Cochrane Controlled Registry of Trials (CENTRAL) for studies comparing Tenecteplase with alteplase in stroke patients
- Looking for absolute risk difference (ARD)
Outcomes:
- Efficacy:
- Functional status at 3 months
- Good functional outcome = mRS 0 to 2
- Excellent functional outcome = mRS 0 to 1
- Recanalization
- Early neurological improvement (ENI)
- Functional status at 3 months
- Safety:
- Cerebral hemorrhage (ICH)
- Symptomatic ICH (sICH)
- 3 month mortality
Inclusion:
- Original studies comparing tenecteplase with alteplase
- Adult patients
- Undergoing thrombolysis for acute ischemic stroke
- Experimental and observational trials
Exclusion:
- None listed
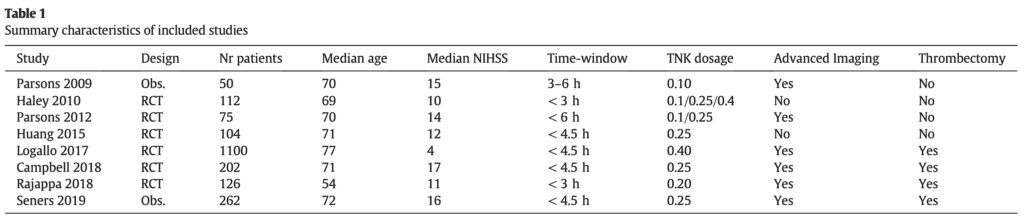
Results:
- 8 trials
- 2031 patients
- 6 randomized trials
- 2 observational trials
-
Efficacy Outcomes:
- No difference in good or excellent functional outcome: ARD 0.07 and 0.03 respectively; p >0.05
- Trend toward improvement with Tenecteplase but not statistically significant
- Tenecteplase showed higher rates of recanalization: ARD 0.11; 95% CI 0.01 to 0.20 (NNT = 9)
- Tenecteplase showed higher rates of early neurological improvement: ARD 0.10; 95% CI 0.02 to 0.17 (NNT = 10)
- No difference in good or excellent functional outcome: ARD 0.07 and 0.03 respectively; p >0.05
-
Safety Outcomes:
- No difference in ICH: ARD -0.02; 0.0.6 to 0.01
- No difference in sICH: ARD 0.00; 95% CI 0.01 to 0.02
- No difference in mortality: ARD 0.00; 95% CI -0.03 to 0.03
Strengths:
- Most comprehensive systematic review and meta-analysis comparing alteplase vs tenecteplase in stroke thrombolysis
- Risk of bias was addressed using the Cochrane Risk of Bias tool
- Publication bias assessed using funnel plots
- Reported heterogeneity of selected studies was low for safety outcomes
- For safety outcomes the results were consistent across all studies without risk of heterogeneity amongst the trials
- PRISMA guidelines were used
Limitations:
- 7 of 8 included trials did not have blinding of participants and personnel which could cause a performance bias
- None of the included trials evaluated transfer times to assess bolus administration prior to transfer (i.e. drip and ship)
- Heterogeneity (variation in study outcomes) was higher for efficacy outcomes across trials
Discussion:
- Also evaluated dosage of Tenecteplase on study outcomes:
- 1mg/kg
- 2 to 0.25mg/kg
- 4 to 0.5mg/kg
- The 0.2 to 0.25mg/kg was superior to alteplase for early neurological improvement (ARD 0.16; 95% CI 0.02 to 0.29) AND there was a trend toward better functional outcomes at 3 months but this outcome was not statistically significant
- 6 of the 8 trials included used advanced perfusion imaging for patient selection and potential for endovascular therapy for large vessel occlusions
- The authors state that there are several studies using tenecteplase in ischemic stroke patients underway to be on the lookout for:
- ATTEST-2, TASTE, NOR-TEST: Thrombolysis up to 4.5hr
- NOR-TEST-2, TWIST: Thrombolysis in wake up stroke
- TEMPO-2: Thrombolysis up to 12hrs
- TASTE-a: Mobile stroke units (DON’T EVEN GET ME STARTED!!!)
- All trials with the exception of NOR-TEST-2 are looking at the 0.25mg/kg dose
- Opening occluded large vessels is more effective with thrombectomy than thrombolysis. Whether we should thrombolyse prior to thrombectomy is an area of debate. This is an area where future research is needed
Author Conclusion: “Tenecteplase is an alternative to alteplase for stroke thrombolysis, with lower cost and a more favourable pharmacokinetic profile.”
Clinical Take Home Point: Tenecteplase appears to be a reasonable alternative to alteplase with a similar safety profile but with the added benefits of helping improve early neurologic improvement and recanalization (surrogates of neurologic outcomes). The 0.2 to 0.25mg/kg dose of Tenecteplase in particular appeared to have the highest early neurological improvement rates and a trend for better functional outcomes at 3 months, however this latter outcome was not statistically significant.
References:
- Oliveira M et al. Tenecteplase for Thrombolysis in Stroke Patients: Systematic Review With Meta-Analysis. AJEM 2021. [Link is HERE]
Post Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami)
The post Tenecteplase for Thrombolysis of AIS? appeared first on REBEL EM - Emergency Medicine Blog.


