
 Background: The optimal management of isolated calf deep vein thrombosis (DVT) is not completely clear, based on the available evidence. The authors of this paper state up to 50% of all lower extremity DVTs are infra-popliteal. Because there is not a lot of robust evidence to guide us on the best diagnostic and therapeutic treatments, a huge variation in practice is seen. To help try and answer these questions the authors of this paper performed the Compression versus Anticoagulant treatment and compression in symptomatic Calf Thrombosis diagnosed by UltraSound – CACTUS Trial.
Background: The optimal management of isolated calf deep vein thrombosis (DVT) is not completely clear, based on the available evidence. The authors of this paper state up to 50% of all lower extremity DVTs are infra-popliteal. Because there is not a lot of robust evidence to guide us on the best diagnostic and therapeutic treatments, a huge variation in practice is seen. To help try and answer these questions the authors of this paper performed the Compression versus Anticoagulant treatment and compression in symptomatic Calf Thrombosis diagnosed by UltraSound – CACTUS Trial.
What They Did:
- Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial
- Enrolled Low-risk outpatients with first acute symptomatic DVT in the calf
- Randomized in a 1:1 fashion to receive low molecular weight heparin nadroparin (171UI/kg SQ QD) or placebo (Saline 0.9% SQ QD) x6 weeks
- Both cohorts wore compression stockings for the duration of the study
Outcomes:
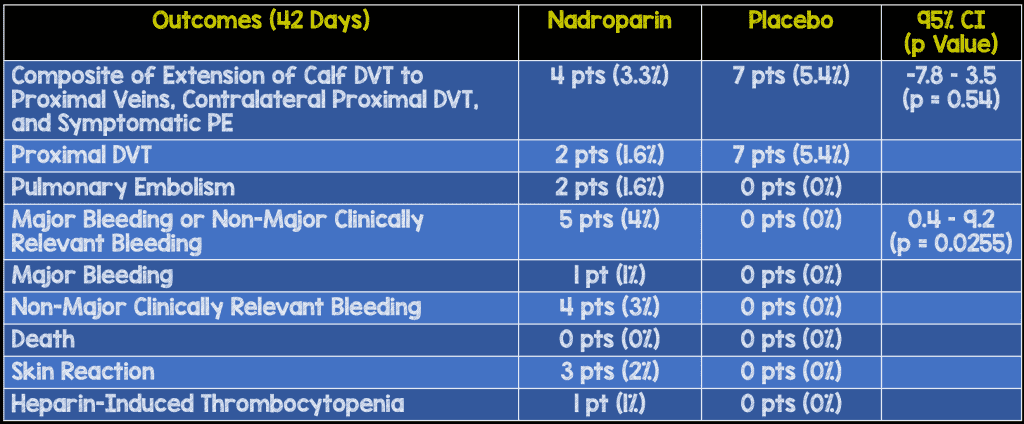
- Primary Efficacy Outcome: Composite of extension of calf DVT to proximal veins, contralateral proximal DVT, and symptomatic pulmonary embolism at day 42
- Secondary Outcomes at day 42 and 90:
- Extension of calf DVT to proximal veins
- Contralateral proximal DVT
- Symptomatic Pulmonary Embolism
- Primary Safety Outcome: Major or clinically relevant non-major bleeding at day 42
- Secondary Safety Outcomes at day 42 and 90:
- Death
- Serious Adverse events
- Post-thrombotic Syndrome
- Quality of life Scores at 1 year
Inclusion:
- Outpatients with a first, acute, symptomatic objectively confirmed calf DVT
Exclusion:
- Thrombus limited to distal superficial veins
- Age <18 years
- Pregnancy
- Previous documented venous thromboembolism
- Calf DVT associated with proximal DVT (popliteal or more proximal) or clinically suspected pulmonary embolism
- Active or recent (<6 months) malignancy
- Another indication for long-term anticoagulation
- Thrombocytopenia (platelet count < 100 x 109 platelets/L)
- Impaired Renal Function (Serum Cr > 180 umol/L or CrCl < 30 mL/min)
- Known hypersensitivity to heparin
- Active Bleeding or a condition associated with high risk of bleeding (Gastric ulcer, Cerebral malignant disease)
- Bodyweight > 115kg or < 40kg
- Already received therapeutic doses of anticoagulants for more than 2 days or ongoing requirement for thromboprophylaxis
- Using daily non-steroidal anti-inflammatory drugs (NSAIDs, aspirin accepted if ≤160 mg/day)
- Enrolled in another trial in previous 30 days
Results:
- 259 patients Enrolled
Strengths:
- Asks a clinically important, patient centered question
- Multicenter Study
- Randomized, Double-Blind, Placebo-Controlled
- Funding sources of the study had no role in study design, data collection, data analysis, data interpretation or writing of the publication
Limitations:
- Authors estimated that they would need 286 patients in each group of the study to have a 90% power to detect a 70% risk reduction in the rate of the primary efficacy outcome, assuming an incidence of the primary outcome of 10% in the placebo group, which was not met in this study
- Only 259 patients enrolled after 6 years, which was 50% of their goal sample size (target sample size was 572 patients)
- Study was terminated early
- Event rates were very low in this already small study, therefore failing to reach any statistical significance
- High risk patients excluded from this study including prior documented venous thromboembolism, inpatients, and patients with active cancer. This makes the results of this study non-generalizable to high-risk patients
- Extensive list of patients excluded from study makes this difficult to apply in clinical practice
- Composite endpoint and all of pieces of composite are not of equal clinical importance
- Nadroparin is not commercially available in the USA
Discussion:
- The American College of Chest Physician guidelines do suggest the option of patients who are low-risk with symptomatic calf DVT (i.e No previous DVT, no Active malignancy) can be safely managed with serial ultrasound testing and no anticoagulant therapy.
- Despite not meeting their original sample study size, the authors advocate against the use of systemic anticoagulation therapy for all patients with calf DVT. One of the major reasons was the lack of serious thromboembolic events in the placebo group (no symptomatic pulmonary embolism at 6 weeks out of 130 patients), combined wth the increased risk of bleeding in the nadroparin group
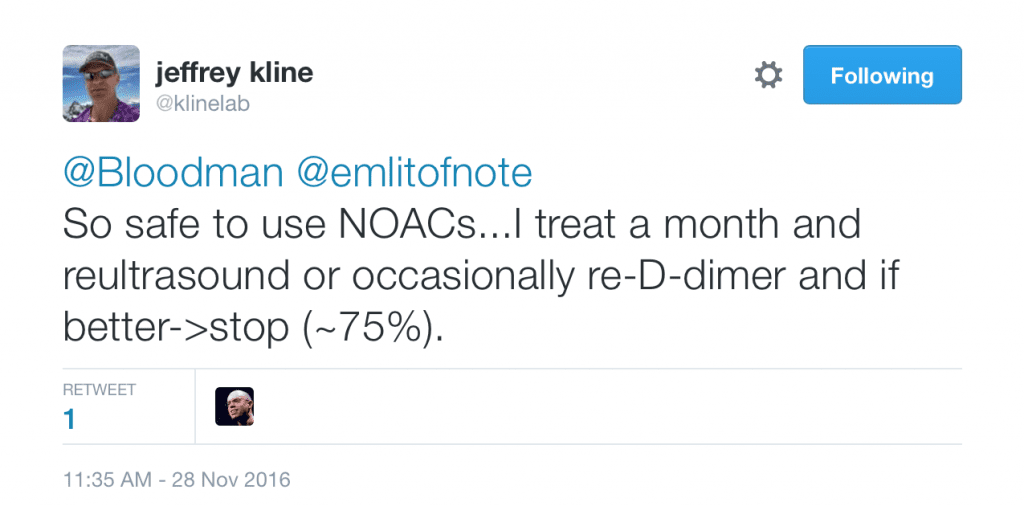
- Jeff Kline also tweeted out a very reasonable management option for isolated distal calf DVTs
Author Conclusion: “Nadroparin was not superior to placebo in reducing the risk of proximal extension or venous thromboembolic events in low-risk outpatients with symptomatic calf DVT, but did increase the risk of bleeding. Avoidance of systematic anticoagulation for calf DVT could have a substantial impact on individual patients from a public health perspective.”
Clinical Take Home Point: This is an underpowered study, that gives us very little guidance on how to treat distal calf DVTs. As there is no clear optimal approach at this time, I would advocate for a shared decision making strategy regarding the use of anticoagulation vs no anticoagulation in isolated low-risk, distal DVTs. With either strategy, close follow up with repeat ultrasound, over the next few days, should be performed to assist with further management and treatment decisions.
References:
- Righini M et al. Anticoagulant Therapy for Symptomatic Calf Deep Vein Thrombosis (CACTUS): A Randomised, Double-Blind, Placebo-Controlled Trial. Lancet Haemotol 2016. S2352 – 3026 (16): 30131 – 4. PMID: 27836513
For More Thoughts on This Topic Checkout:
- Rob Orman on ERCast: The Problem with Calf Clots
- Ryan Radecki on EM Lit of Note: No Guidance for Calf Clots from CACTUS
- Jacob Avila and Mike Mallin on Ultrasound Podcast: Ultrasound for Deep and Superficial vein clots part 1. Calf Clots?!?!
- David Wonnacott at CanadiEM: Isolated Distal DVT – Diagnosis and Management
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)
The post The CACTUS Trial: Anticoagulation for Symptomatic Calf Deep Vein Thrombosis? appeared first on REBEL EM - Emergency Medicine Blog.
