
 Background/Introduction: The commonly cited duration of antibiotic therapy (7-14 days) for community-acquired pneumonia (CAP) has historically been based on arbitrary timelines extrapolated from a desire to prevent treatment failures and avoid under-treatment rather than evidence-based medicine. Even the original literature on the treatment of pneumococcal pneumonia all reported effective duration of antibiotic treatment to be 2 to 4 days:
Background/Introduction: The commonly cited duration of antibiotic therapy (7-14 days) for community-acquired pneumonia (CAP) has historically been based on arbitrary timelines extrapolated from a desire to prevent treatment failures and avoid under-treatment rather than evidence-based medicine. Even the original literature on the treatment of pneumococcal pneumonia all reported effective duration of antibiotic treatment to be 2 to 4 days:
- 1943 Keefer et al. 500 patients with the majority recovering in 2-3 days.
- 1944 Dawson et al. 100 patients with the majority recovering within 2 days.
- 1944 Tillet et al. 31 patients with the majority recovering in 3-4 days.
Then in 1945, Meads et al. published their data in the NEJM where they administered antibiotics to 44 patients until there was “definitive clinical improvement and the temperature had remained below 100°F for 12 hours…then given for another two to three days.” The vast majority of patients recovered within 3 to 4 days but there were 2 patients which the authors mistakenly identified as failure of treatment. Their rationale for continuing to treat beyond the resolution of clinical symptoms and fever was driven by a desire to prevent these “treatment failures” as suggested by the author. Medical historians have suggested that this publication seems to have been the evidence base for the prolonged (7-14 days) duration of treatment for CAP that became the standard of care for ensuing decades. The problem is that until recently, the optimal duration of therapy for CAP has not been rooted in evidence-based medicine.
Similarly, in the pediatric population, not only the antibiotic duration but also the optimal dosages for the treatment of CAP have gone through multiple iterations and are still being studied. Children younger than 5 years of age that come to the Emergency Department with respiratory infectious symptoms commonly receive antibiotics as neither chest radiography nor inflammatory biomarkers reliably differentiate who best benefits from antibiotic therapy. This lack of predictive testing indicates children presenting to the Emergency Department with signs of CAP will continue to be prescribed antibiotics.
Thus, promoting the selection of optimal antibiotic dosing and length of therapy is pivotal within an antimicrobial stewardship framework to reduce the frequency of adverse events, the development of resistance, as well as healthcare costs. This study, along with the recently published SAFER trial aims to further our knowledge and understanding of optimal antibiotic dosing and duration in the pediatric population.
Paper: Bielicki J et al. Effect of Amoxicillin Dose and Treatment Duration on the Need for Antibiotic Re-treatment in Children With Community-Acquired Pneumonia: The CAP-IT Randomized Clinical Trial. JAMA. 2021. PMID: 34726708
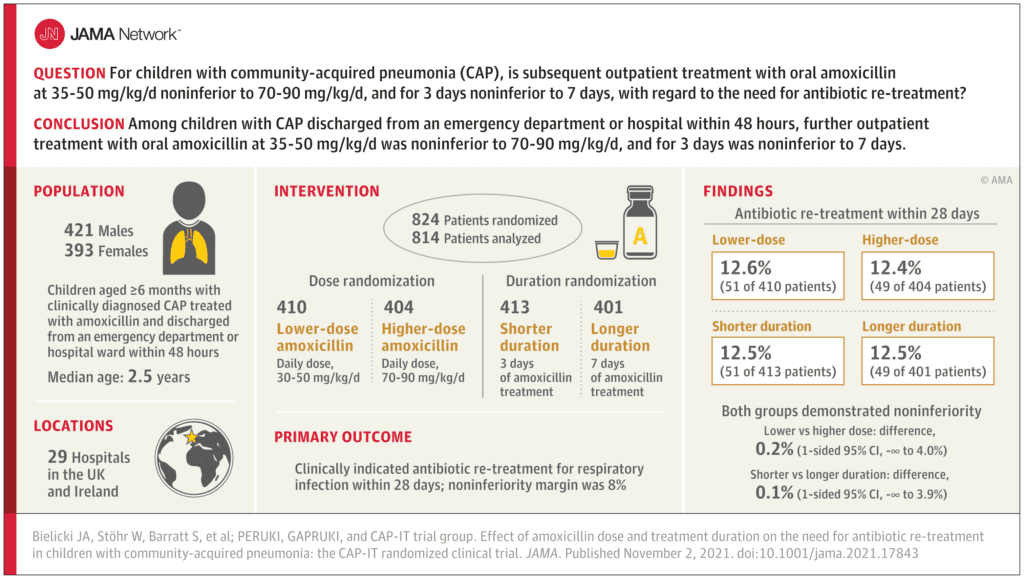
Clinical Question: Is lower dose amoxicillin (35-50 mg/kg/day) noninferior to high dose amoxicillin (70-90 mg/kg/day) and is a 3-day treatment duration noninferior to a 7-day treatment duration for CAP in children discharged from the Emergency Department, Observation Unit, or the Inpatient setting (within 48-hours).
What They Did:
- Randomized, blinded, placebo-controlled, multicenter noninferiority clinical trial in 28 hospitals in the UK and 1 in Ireland
- Patients randomized to receive amoxicillin in following groups:
- 30-50 mg/kg/d for 3 days
- 30-50 mg/kg/d for 7 days
- 70-90 mg/kg/d for 3 days
- 70-90 mg/kg/d for 7 days
- Additionally, a nasopharyngeal swab for Streptococcus pneumoniae carriage and resistance was taken at enrollment.
- After discharge, follow-up data was collected at prespecified time points assessing for CAP symptoms, adverse events, trial medication adherence, and any non-trial antibiotic prescriptions.
- At day 28, a repeat nasopharyngeal swab for Streptococcus pneumoniae was collected.
- The trial was designed to demonstrate noninferiority of lower dose compared with higher dose, and shorter duration compared with longer duration.
Outcomes:
- Primary Outcomes: Clinically indicated antibiotic re-treatment for respiratory infection within 28 days after randomization with noninferiority margin of 8%
-
Secondary Outcomes:
- Severity/duration of 9 parent-reported CAP symptoms
- Amoxicillin-related clinical adverse events
- Adherence to trial medication
- Phenotypic penicillin nonsusceptibility or resistance in nasopharyngeal S Pneumoniae isolates
Inclusion Criteria:
- Children older than 6 months of age
- Weighed 6 to 24 kg
- Clinically Diagnosed with CAP as defined by British Thoracic Society:
- Parent/guardian reported cough w/in previous 96 hours
- Measured temperature of 38°C or guardian-reported fever within previous 48 hours
- Signs of labored or difficult breathing without focal chest signs
- Treatment with Amoxicillin monotherapy on discharge from hospital ED, observational unit, or inpatient ward
Exclusion Criteria:
- Uninterrupted prior B-lactam antibiotic treatment for more than 48 hours or any prior non-B-lactam treatment
- Severe underlying chronic disease
- Any contraindication to amoxicillin including allergy
- Bilateral wheezing without focal chest signs
- Complicated pneumonia:
- Defined as: Signs of sepsis, local parenchymal or pleural complications
Results:
- 814 patients enrolled and randomized into 1 of 4 groups
- 52% of patients were male
- Median age = 2.5 years
- 73% discharged directly from the ED
- 27% had an inpatient stay of <48hrs
-
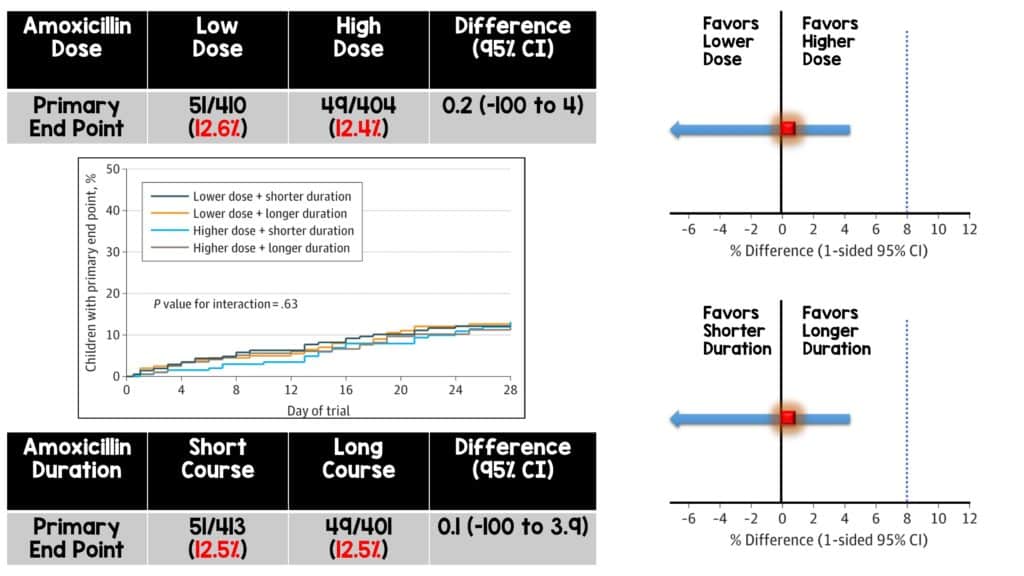
Primary Outcome
- Lower vs Higher Amoxicillin Dose Group: The primary outcome occurred in 12.6% of patients treated with lower amoxicillin dose vs 12.4% of patients treated with higher amoxicillin dosing.
- Shorter vs Longer Amoxicillin Duration Group: The primary outcome occurred in 12.5% of patients treated with a 3-day treatment course vs 12.5% of patients treated with a 7-day treatment course.
- Both groups demonstrated non-inferiority with no significant interaction between dose and duration.
-
Secondary Outcomes
- Severity/duration of 9 parent-reported CAP symptoms: No significant difference between groups by dose or duration in 7/9 measured outcomes
- Cough and sleep disturbed by cough persisted longer in shorter-vs-longer duration (12d vs 10d; HR 1.2; 90% CI 1.0 to 1.4; p = 0.04)
- No significant association between dose or duration of amoxicillin and severity of cough symptoms
- Amoxicillin-related clinical adverse events: In total, 5% of participant experienced a serious adverse but only one classified as related to trial medications.
- Diarrhea was reported in 44% of children, rash in 24%, and oral thrush in 7%.
- Adherence to trial medication: Active trial medication was discontinued early by 6% of participants while 14% took fewer doses or a lower volume than prescribed.
- Children randomized to 3 days of amoxicillin were more likely to complete their full treatment course compared with those randomized to a 7-day course (98% vs 91%).
- Phenotypic penicillin nonsusceptibility or resistance in nasopharyngeal S Pneumoniae isolates: No significant difference in day 28 pneumococcal carriage or penicillin nonsusceptibility according to the dose or duration of amoxicillin
- Severity/duration of 9 parent-reported CAP symptoms: No significant difference between groups by dose or duration in 7/9 measured outcomes
Strengths:
- Multi-center, randomized, blinded study design
- Primary and secondary endpoints are patient-oriented
- Adds further information to a question with inconsistent previous research during a time when antibiotic stewardship is being recognized as vitally important in the fight against the global emergence of antibiotic resistance.
- This is the first study examining both dosage as well as duration of antibiotics for pediatric CAP.
Limitations:
- Children commonly exhibit a mixed pattern of disease (bacterial, viral, ect) and it is likely that a proportion of patients may have been infected with non-bacterial illness to begin with.
- Symptoms that informed the primary and secondary outcomes were recorded using a diary given to parents upon discharge. This has the potential for measurement bias as well as possible recall bias depending on how and when parents recorded symptoms.
- The trial was not powered to investigate non-inferiority of lower dose and shorter duration of amoxicillin therapy in the subgroup of children discharged after an inpatient stay.
- Total treatment duration for children initially admitted to the hospital was not taken into consideration and authors state that the optimal treatment duration may differ in those requiring prolonged intravenous antibiotic treatment as inpatients.
- Authors explicitly state that their findings should not be generalized to children with very severe disease.
Discussion:
- This is an important study that is attempting to clarify the evidence-based nature of optimal dosing and duration of antibiotic use for pediatric CAP. The most recent CAP pediatric guidelines published in 2011 by the Pediatric Infectious Disease Society and the Infectious Disease Society of America states that “most experts believe that, when pneumococcal pneumonia is suspected, and oral therapy is appropriate, high dose amoxicillin is preferred.” They mention that in the early 1970’s, the vast majority of Streptococcus pneumoniae isolates were highly susceptible to dosing regimens of 40-45 mg/kg/day but with the development of widespread pneumococcal resistance in the 1990’s and the emergence of antibiotic-resistant serotype 19A strains, 90 mg/kg/day dosing is more likely to be successful. Thus, the guidelines currently endorse empiric treatment of CAP with amoxicillin at a dose of 90 mg/kg/day and state that treatment courses of 10 days have been best studied. The evidence summary makes a mention of shorter 3-day courses in the developing world but states that this cannot be reliably extrapolated to developed countries at the time of publication. This study, along with the recent SAFER trial and other trials in adult populations are attempting to fill the evidence-base void when it comes to optimal antibiotic usage within the framework of antibiotic stewardship.
- The authors of this study note that for pediatric patients discharged from the ED or an inpatient unit with CAP, antibiotic re-treatment rates for respiratory tract infections within 4 weeks were non-inferior among those randomized to lower vs higher dose amoxicillin and among those randomized to a 3-day vs a 7-day course of treatment.
- They argue that there is emerging evidence for the use of shorter and lower dosing for the treatment of CAP with similar efficacy while noting that the adverse event profile did not differ based on the treatment regimens and subsequent pneumococcal resistance was not acquired. But the big question is whether these study participants were truly afflicted with a bacterial pneumonia to begin with. Their criteria for diagnosis of CAP does not reliable differentiate between viral and bacterial etiologies of CAP, but as the authors note, at this current time, neither do radiographic or laboratory biomarkers. Thus, it is possible that a proportion of patients were treated with antibiotics when they had a viral infection, and this may have affected the results of this trial.
- Another vital take-home point to remember is that when looking at the secondary outcomes, cough persisted longer in the shorter vs the longer duration group as well as sleep disturbed by cough. This may have implications for families with sick children who value more expedient resolution of these symptoms. So, this needs to we weighed against the potential side effect profile and compliance of different treatment regimens.
- Overall, this study trends towards endorsing the use of lower dosage and shorter duration of amoxicillin for the treatment of pediatric CAP but it needs to be interpreted in setting of its limitations. More extensive trials are needed to support a definitive practice change.
Author Conclusions:
“Among children with CAP discharged from an ED or hospital ward (within 48 hours), low-dose outpatient oral amoxicillin was noninferior to high dose, and 3-day duration was noninferior to 7 days, with regard to need for further antibiotic retreatment.”
Clinical Take Home Points:
Investigators found that lower dosing and treatment duration of amoxicillin is non-inferior for the treatment of pediatric CAP when compared to higher dosing and treatment duration. However, these findings need to be interpreted in the context of whether antibiotics would have helped at all given the realistic probability of high rates of viral illness in this patient population. Also disease severity, treatment setting, prior antibiotics, and what an acceptable non inferiority margin is require consideration in interpreting these findings. Additional studies are necessary but this study along with the SAFER trial have the potential to move the needle in terms of supporting the use of lower dose and duration of amoxicillin therapy for pediatric CAP.
JAMA Visual Abstract:
Guest Post By:

Alex Hamidi
PGY-3 Emergency Medicine Resident
Inspira Medical Center
Vineland, NJ
References:
- Keefer C et al. Penicillin in the treatment of infections: a report of 500 cases. JAMA. 1943. PMID: Link
- Dawson M et al. The clinical use of penicillin: observations in one hundred cases. JAMA. 1944. PMID: Link
- Tillet W et al. The Treatment of Lobar Pneumonia and Pneumococcal Empyema with Penicillin. Bull NY Acad Med. 1944. PMID: 19312369
- Meads M et al. Treatment of pneumococcal pneumonia with penicillin. NEJM. 1945. PMID: Link
For More Thoughts on This Topic Checkout:
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The CAP-IT Trial: Amoxicillin Dose and Duration in Children with Community-Acquired Pneumonia appeared first on REBEL EM - Emergency Medicine Blog.
