 Background: On December 23, 2021, the FDA granted molnupiravir emergency use authorization for the treatment of patients with Covid-19. Molnupiravir is a nucleoside analog that inhibits SARS-CoV-2 replication and triggers viral RNA mutagenesis. Until the release of oral antivirals, clinicians had few treatment options for outpatient management at their disposal.
Background: On December 23, 2021, the FDA granted molnupiravir emergency use authorization for the treatment of patients with Covid-19. Molnupiravir is a nucleoside analog that inhibits SARS-CoV-2 replication and triggers viral RNA mutagenesis. Until the release of oral antivirals, clinicians had few treatment options for outpatient management at their disposal.
This paper assesses the efficacy of molnupiravir in the management of nonhospitalized patients with mild to moderate Covid-19 who are at risk for developing severe disease.
Paper: Jayk Bernal A et al. Molnupiravir for Oral Treatment of Covid-19 in Nonhospitalized Patients [published online ahead of print, 2021 Dec 16]. NEJM 2021. PMID: 34914868
Clinical Question: Is molnupiravir superior to placebo in preventing hospitalization and death through day 29 in patients with mild to moderate Covid-19?
What They Did:
- Phase 3 component of MOVe-Out Trial
- Double-blind, parallel-group, randomized, placebo-controlled trial
- Patients enrolled May 6, 2021 – October 2, 2021
- 107 sites in 20 countries
- Eligible patients were randomly assigned to receive either molnupiravir or placebo.
- Randomization was stratified in blocks of four according to the time since onset of signs or symptoms (≤3 days vs. >3 days)
- Prespecified interim analysis once 50% of the target enrollment followed through day 29.
- An independent data monitoring committee recommended that recruitment be stopped early for benefit.
Population:
Inclusion Criteria:
-
Non Hospitalized adults with mild or moderate Covid-19
-
Moderate Covid-19:
- Shortness of breath with exertion, respiratory rate ≥20 to <30 breaths per min and/or heart rate ≥90 to <125 beats per minute
- AND SpO2 >93% on room air or on supplemental oxygen for a reason other than Covid-19, which has not increased due to Covid-19. OR patient was receiving ≤4 liters/min supplemental oxygen for Covid-19, regardless of SpO2, but was not previously on supplemental oxygen.
- AND No shortness of breath at rest, respiratory failure, shock, or multi-organ dysfunction/failure.
- SARS-CoV-2 infection laboratory-confirmed ≤5 days
-
Moderate Covid-19:
- The onset of signs or symptoms ≤5 days
- At least one sign or symptom of Covid-19
-
At least one risk factor for the development of severe illness from Covid-19
- Age >60 years
- Active cancer
- Chronic kidney disease
- Chronic obstructive pulmonary disease (COPD)
- Obesity, (body-mass index ≥30)
- Serious heart conditions (heart failure, coronary artery disease, or cardiomyopathies)
- Diabetes mellitus
Exclusion Criteria:
- Anticipated need for hospitalization for Covid-19 within the next 48 hours
- Dialysis or GFR <30 ml/min/1.73 m2
- Pregnancy
- Unwilling to use contraception during the intervention and 4 days after completion
- Severe neutropenia (absolute neutrophil count of <500/mL)
- Platelet count below 100,000/µL
- SARS-CoV-2 vaccination
Intervention:
- Molnupiravir 800 mg (four 200-mg capsules) twice daily for 5 days
Control:
- Placebo (four identical pills) twice daily for 5 days
Outcome:
Primary Outcome: Composite of:
- Hospitalization for any cause: ≥24 hours of acute care in a hospital or any similar facility
- Death through day 29
Secondary Outcomes:
-
Time to resolution of COVID-19 sign/symptom through 29 days
- The number of days from randomization to the first of 3 consecutive days of resolution (without subsequent relapse by day 29)
-
Time to progression of COVID-19 sign/symptom through 29 days
- The number of days from randomization to the first of 2 consecutive days of worsening
-
Signs and Symptoms
- Loss of smell
- Fatigue
- Shortness of breath
- Loss of taste
- Sore throat
- Diarrhea
- Nasal congestion (stuffy nose)
- Chills Cough
- Subjective fever
- Headache
- Bodyache
- Rhinorrhea (runny nose)
- Nausea
- Vomiting
-
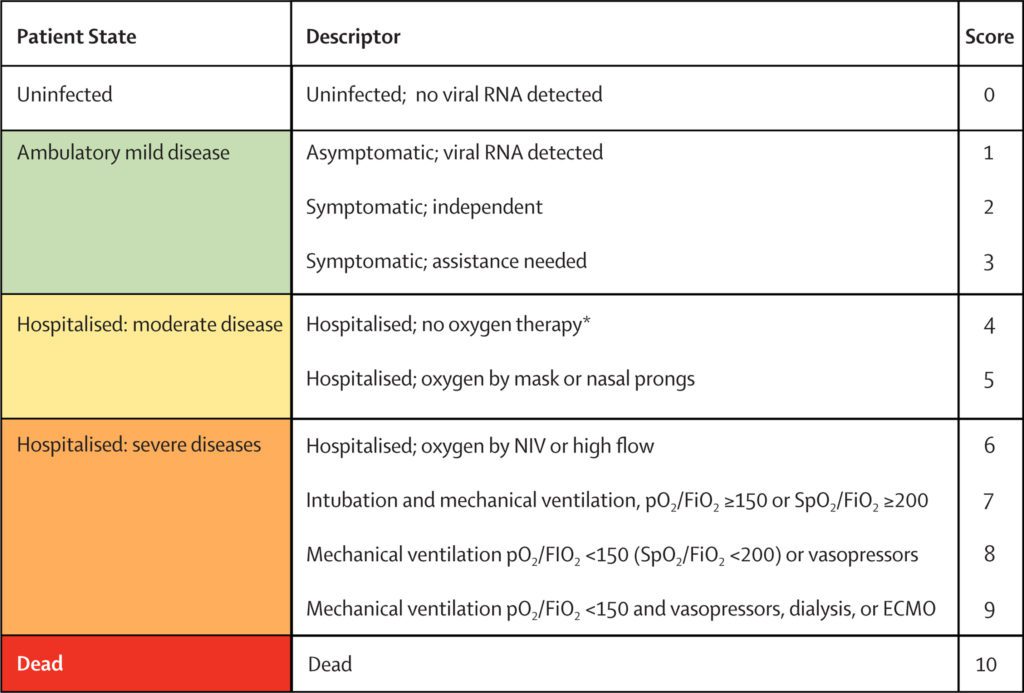
World Health Organization (WHO) 11-point outcomes score on a scale through 29 days.
- WHO outcome scale: an 11-point ordinal score that categorizes clinical progression.
- The score ranges from 0 to 10, with a higher score indicating clinical progression.
- Mean changes in SARS-CoV-2 viral load from baseline.
- Image: WHO Working Group on the Clinical Characterisation and Management of COVID-19 infection. A minimal common outcome measure set for COVID-19 clinical research [published correction appears in Lancet Infect Dis. 2020 Oct;20(10):e250]. Lancet Infect Dis. 2020;20(8):e192-e197.
Primary Safety Outcome:
- Incidence of Adverse events
Results:
-
1433 patients were randomized
- 716 patients assigned to the molnupiravir group
- 717 patients assigned to the placebo group
-
1408 patients included in the modified intention-to-treat analysis
- 709 patients from the molnupiravir group
- 699 patients from the placebo group
Primary Outcome:
- In the interim analysis, the molnupiravir group had a lower risk of hospitalization or death through day 29, 7.3% (28/385) compared to 14.1% (53/377) in the placebo group.
- Treatment difference of 6.8% (95% CI −11.3 to −2.4; P = 0.001).
- However, the treatment difference was much lower in the full trial.
-
Molnupiravir group: 6.8% (48/709 participants) participants were hospitalized or died through day 29
- One death was reported in the molnupiravir group (29-day all-cause mortality, 0.1%)
-
Placebo group: 9.7% (68/699 participants) participants were hospitalized or died through day 29
- 9 deaths in the placebo group (29-day all-cause mortality, 1.3%)
- Treatment Difference of 2.9%; (95% CI, −5.9 to −0.1)
- Treatment with molnupiravir resulted in 1 less death and hospitalization for every 34 patients treated. (Number Needed to Treat = 34)
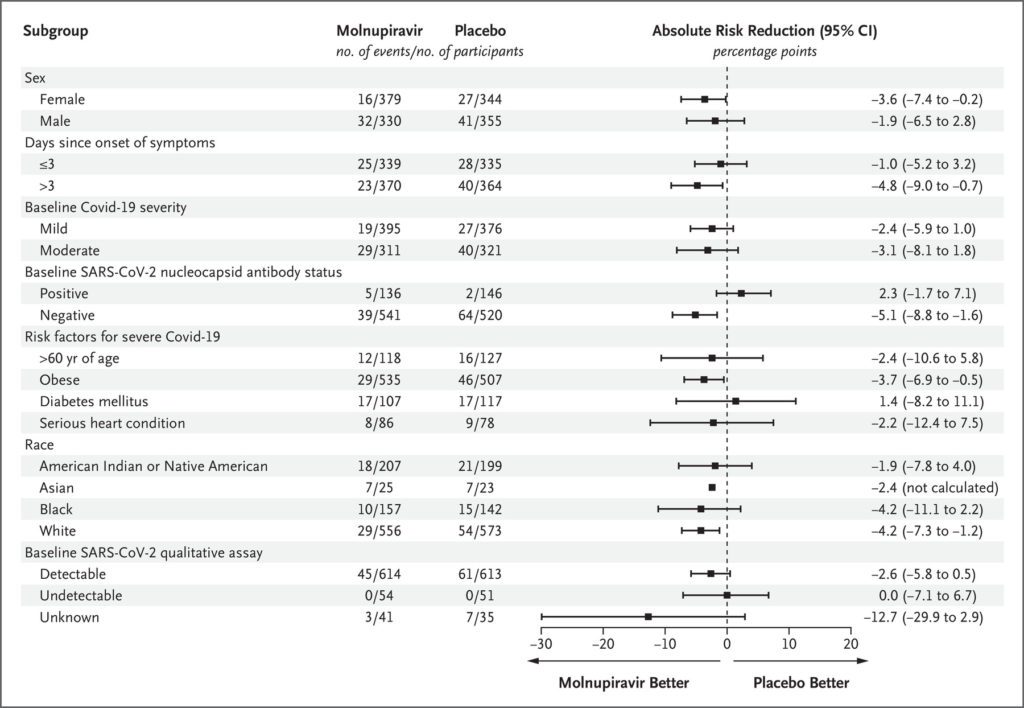
Secondary Outcomes:
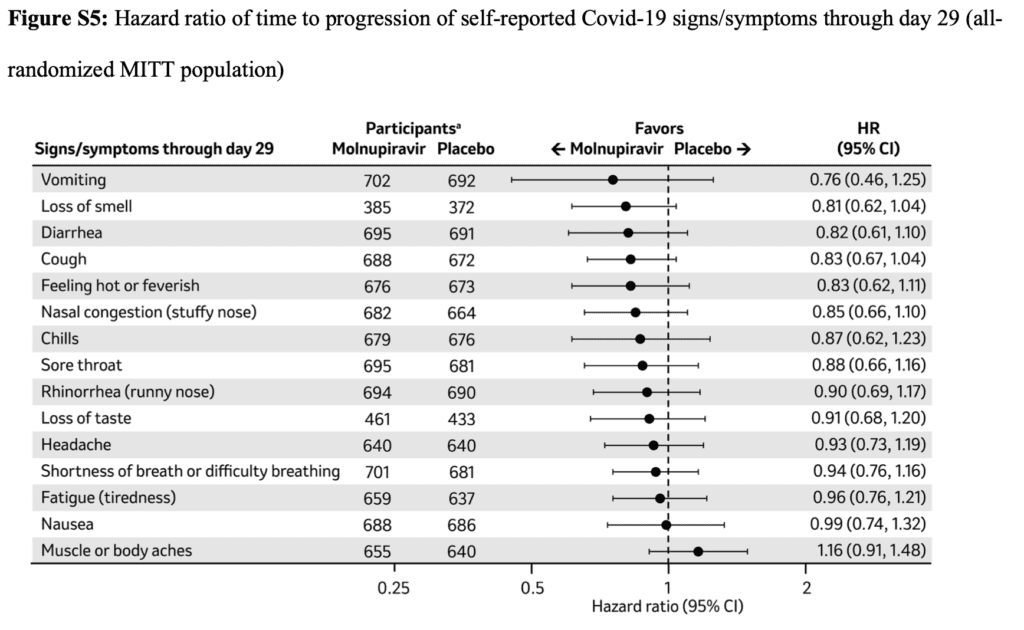
- For most Covid-19 signs and symptoms, sustained resolution was more likely in the molnupiravir group.
- Progression of signs or symptoms was less likely in the molnupiravir group than in the placebo group.
- Based on the WHO Clinical Progression Scale, more patients in the molnupiravir group showed improved outcomes by day 5, with the largest differences observed by days 10 and 15
- Molnupiravir treatment was associated with larger reductions from baseline in mean viral load than placebo at days 3, 5.
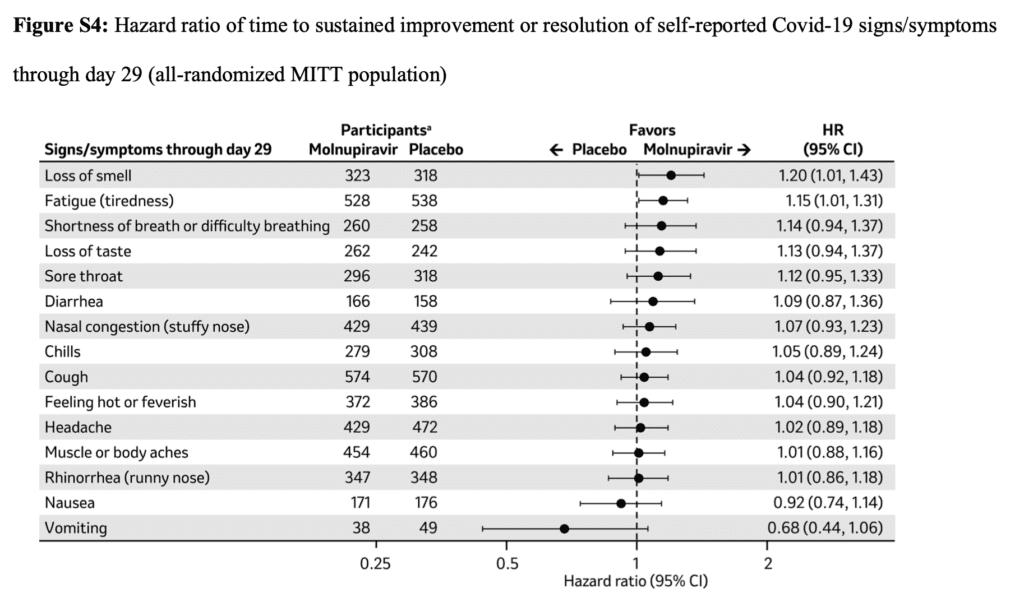
Primary Safety Outcomes:
-
30.4% in the molnupiravir group had at least one adverse event.
- 8.0% related to trial regimen
-
33.0% in the placebo group had at least one adverse event.
- 8.4% related to trial regimen
- No deaths from adverse events were attributed to the trial regimen in either group.
-
The most frequently reported adverse events: occurred in ≥2% of participants in either group:
- Covid-19 pneumonia (6.3% of participants in the molnupiravir group vs. 9.6% in the placebo group)
- Diarrhea (2.3% vs. 3.0%)
- Bacterial pneumonia (2.0% vs. 1.6%)
- Worsening of Covid-19 was reported as an adverse event in 7.9% vs. 9.8%.
-
The most frequently reported adverse events: occurred in ≥1% of participants in either group deemed to be related to the trial regimen:
- Diarrhea (1.7% vs. 2.1%)
- Nausea (1.4% vs. 0.7%)
- Dizziness (1.0% vs. 0.7%)
- One participant each in the molnupiravir and placebo groups had a postbaseline platelet count below 50,000/µL.
Strengths:
- The primary outcome was clinically relevant and patient-oriented.
- A double-blind, parallel-group, randomized, placebo-controlled trial minimizes bias.
- Pills were identical minimizing potential for unblinding and bias.
- International study with enrollment at more than 100 sites in 20 countries increased the external validity.
- The study population was representative of a real-world population as nearly all patients had at least one major risk factor for severe illness in Covid-19.
- Missing data reported as meeting the primary endpoint
- Definition of moderate Covid-19 allowed for sick people.
- Nearly half, 44.5%, had moderate covid.
-
Compliance was excellent in both arms.
- Among those who received molnupiravir or placebo, most (95.2% in the molnupiravir group and 94.7% in the placebo group) received at least 9 doses.
Limitations:
- Funded by Merck. Trials funded by pharmaceutical companies are at increased risk for bias.
- The primary outcome is a composite and the two components (death and hospitalization) are not equally important
- Some secondary outcomes were disease-oriented.
- An independent review board decided to stop the trial early for benefit.
- There were no predetermined stop criteria listed.
-
Important information is listed only in the supplement.
- Definition of moderate Covid-19
- Some baseline characteristics like region and ethnicity
- Results for secondary outcomes
- 49.6% of patients were of Hispanic ethnicity and 46.2% patients were enrolled in Latin America which decreases generalizability.
- Vaccinated patients were excluded.
- Convenience sample. It’s unclear how many people were eligible but not randomized contributing to selection bias.
- More women were randomized to the molnupiravir arm in the interim analysis.
- The trial is underpowered based on actual incidence rates.
Discussion:
-
More women were randomized to the molnupiravir group than to the control group
- This variance was larger in the interim analysis and likely contributed to the larger discrepancy in treatment difference.
- Globally more men have died than women, and the case fatality ratio is 2.4x higher in men compared to women.
- If women are less at risk than men for death and hospitalization this could potentially bias the results in favor of molnupiravir.
- Post hoc analysis adjusted for sex demonstrated a similar treatment difference of 2.8% with molnupiravir over placebo.
- However, the confidence interval crossed zero (95% CI, −5.7 to 0.1) indicating no statistical significance.
-
Truncated RCTs
- Trials stopped early for an apparent benefit are at risk for overestimating results.
- An independent review board made the decision during a predetermined interim analysis.
- However, there were no predetermined stopping rules.
- At the time of interim analysis, investigators recruited 92% of the planned study population.
- However, the study is still considerably underpowered.
- The investigators assumed incidence rates of 6% in the molnupiravir group and 12% in placebo. But, the actual incidence rates were 6.8% in the molnupiravir group and 9.7% in the placebo group.
- The trial would require a sample size of 5430 patients for appropriate power, based on the actual incidence rates with α of 0.025 and power of 95%.
-
The primary outcome is a composite of death and hospitalization.
- Investigators often use composite outcomes to decrease the sample size required to show statistical significance.
- Physicians might mistakenly assume that the effect of the intervention applies equally to all components of the composite endpoint.
- All components are not equal, and patients are sure to prioritize death over hospitalization.
- The components did not occur at the same frequency, and hospitalization drove the outcome in this trial.
-
Publication Supplement
- Lots of important information was only available in the supplement.
- For example, the authors curiously left out the definition of moderate Covid-19.
- Also left out was that approximately 50% of patients were recruited in Latin America and approx 50% were of Hispanic descent, affecting generalizability.
- A large portion of information regarding secondary outcomes was only available in the supplement.
- Many clinicians don’t have time to read a 10-page article, let alone a 30-page supplement.
-
Moderate Covid-19
- The definition of moderate Covid-19 was broad and allowed for some potentially sick patients.
- 44.5% of patients enrolled had moderate Covid-19.
- Patients with moderate Covid-19 were permitted to use as much as 4 LPM of supplemental oxygen.
- However, many clinicians might admit hypoxic patients on supplemental oxygen therapy, thus excluding them from treatment with molnupiravir.
-
Important Exclusions
- Vaccinated patients were excluded. There is little doubt that vaccinated patients will want treatment when afflicted with coronavirus. Although at this point, it’s unclear if molnupiravir will confer any benefit to patients who may already have antibodies against Covid-19.
- In subgroup analysis of patients with positive nucleocapsid antibodies, treatment benefit favored placebo over molnupiravir.
- The study protocol also did not allow for concomitant treatment with remdesivir or monoclonal antibody therapy. It makes sense in a clinical trial to avoid confounding any possible results. But in clinical practice, does this matter, and can patients get multiple therapies?
- Admitted patients were excluded from treatment, but some may be eligible for treatment based on the definition of moderate Covid-19.
-
Teratogenic
- Based on animal models, molnupiravir maybe be teratogenic and is not recommended during pregnancy.
- Women of childbearing age should use contraception for four days after completion.
- Men should use contraceptives for at least 3 months after treatment, which is a huge compliance risk.
Author’s Conclusion: “In this trial, oral molnupiravir was found to be effective for the treatment of Covid-19, without evident safety concerns, when initiated within 5 days after the onset of signs or symptoms in this population of nonhospitalized, unvaccinated adults who were at risk for progression to severe disease.”
Our Conclusion: We agree with the author’s conclusion. Oral molnupiravir decreased the composite outcome of death and hospitalization at 29 days by 30%. However, the trial is markedly underpowered and some benefits may be due to gender differences in the experimental and placebo groups. In addition, molnupiravir appears safe and has few exclusions. Finally, teratogenic concerns are a major issue and compliance with contraception recommendations may be difficult to maintain particularly for men.
Clinical Bottom Line:
- Molnupiravir is approved under emergency use authorization to treat mild to moderate Covid-19.
- Clinicians should start molnupiravir within five days of symptom onset.
- Restrict molnupiravir to patients at high risk for death and hospitalization until it is more widely available.
- While unvaccinated patients were excluded from the study, we should also prescribe molnupiravir to this population.
For More on This Topic Checkout
- ALiEM: Oral Antivirals for Treatment of Mild-Moderate COVID-19 Infection
- EMRAP: COVID-19 Oral Antivirals
References:
- O’Shaughenessy J. Food and Drug Administration. Molnupiravir Emergency Use Authorization 108. December 23, 2021. [Link is here ]
- Derek M. Griffith, PhD1; Garima Sharma, MD2; Christopher S. Holliday, PhD, MPH3; Okechuku K. Enyia, MPH4; Matthew Valliere, MPA5; Andrea R. Semlow, MS, MPH1; Elizabeth C. Stewart, DrPH, MSPH1,6; Roger Scott Blumenthal, MD2. [Link is here]
Post-Peer Reviewed By: Anand Swaminathan, MD (Twitter: @EMSwami) and Salim R. Rezaie, MD (Twitter: @srrezaie)
The post The MOVe-Out Trial: Molnupiravir – Oral Antivirals for Management of Covid-19? appeared first on REBEL EM - Emergency Medicine Blog.