
 Background: Many physicians struggle with monitoring accurate continuous blood pressures, cardiac output, and response to fluids in patient resuscitation. Also, due to the invasive nature of most methods presently available (i.e. arterial lines, etc) few patients get this monitoring. Ultrasound has been an amazing addition to our armamentarium, but many, I am sad to say, still don’t feel comfortable with this modality. Recently, finger cuff, non-invasive technology was brought to my attention by Bob Frolichstein (Twitter: @frolichstein), one of my colleagues in San Antonio, TX. Specifically, it has been stated that, finger cuff technology, allows hemodynamic monitoring with both BP and CO continuously available in patients without the need for an arterial line.
Background: Many physicians struggle with monitoring accurate continuous blood pressures, cardiac output, and response to fluids in patient resuscitation. Also, due to the invasive nature of most methods presently available (i.e. arterial lines, etc) few patients get this monitoring. Ultrasound has been an amazing addition to our armamentarium, but many, I am sad to say, still don’t feel comfortable with this modality. Recently, finger cuff, non-invasive technology was brought to my attention by Bob Frolichstein (Twitter: @frolichstein), one of my colleagues in San Antonio, TX. Specifically, it has been stated that, finger cuff technology, allows hemodynamic monitoring with both BP and CO continuously available in patients without the need for an arterial line.
History: Finger cuff technology was first introduced in the 1980s. The simplistic way this works is finger artery diameter is measured and monitored by an infrared photo-plethysomograph. Brachial artery pressure is reconstructed from finger artery pressures to calculate the blood pressure and a device calculation from the reconstructed brachial artery pressure to determine the CO. Analysis of the finger artery is repeated on a regular basis (i.e. every 30 – 60 seconds) following changes in physiological states resulting in a continuous, non-invasive BP and CO monitoring.

What is the Evidence for Use of Finger Cuff Technology:
- Anesthesia Literature
- Vos JJ et al [2]: 112 patients undergoing elective general surgery showed non-inferiority when compared to the gold standard arterial line and more traditional standard intermittent arm cuff blood pressure cuffs.
- Stens J et al [3]: Observational study of 54 patients undergoing non-thoracic surgery with positive pressure mechanical ventilation were placed in supine steady state and Trendelenburg positions. Nexfin showed reasonable reflections in alterations in pulse pressure variation and stroke volume variation
- Vos JJ et al [4]: Observational study of 90 patients undergoing general surgery receiving a 500mL fluid bolus showed reasonable fluid responsive prediction
Is there EM Evidence for Finger Cuff Technology in the Acute Setting:
Study #1 [5]:
What They Did:
- Prospective Observational, Exploratory Convenience Study of Acutely Ill Adults
- 40 acutely ill ED patients with broad ranges of BP and HR underwent simultaneous monitoring using interval standard automated ED devices and continuous finger cuff technology (Nexfin) at baseline and every 15 minutes over a period of 2 hours
Outcomes:
- Comparison of accuracy of BP and HR measurements using continuous finger cuff technology vs simultaneous measurements with interval standard automated ED devices
Inclusion:
- Acutely Ill ED patients (i.e. Level 1 severity of illness)
- Acute shortness of breath thought to be caused by congestive heart failure and/or chronic obstructive lung disease
- Probable acute stroke syndromes
- Suspicion of sepsis
- Abnormal BP (SBP >180mmHg or <100mmHg)
Exclusion:
- <18 years of age
- Cardiopulmonary arrest
- Documented to have a ST-segment elevation myocardial infarction
- Pregnancy
- Excessive agitation making monitoring difficult to accomplish
Results:
- 40 patients
- 15 had acute dyspnea
- 11 had acute stroke-like symptoms
- 3 had suspected sepsis
- 8 with SBP >180mmHg or <100mmHg
- Diastolic Blood Pressure (>300 Readings)
- Mean Intermittent Device: 77.19 (Range: 29.00 – 151.00)
- Mean Nexfin: 78.43 (Range: 35.00 – 157.00)
- Pearson Correlation Coefficient 0.75
- Systolic Blood Pressure (>300 Readings)
- Mean Intermittent Device: 143.56 (Range: 69.00 – 230.00)
- Mean Nexfin: 142.69 (Range: 48.00 – 263.00)
- Pearson Correlation Coefficient 0.83
- MAP (>300 Readings)
- Mean Intermittent Device: 99.31 (Range: 47.00 – 169.33)
- Mean Nexfin: 101.08 (Range: 40.00 – 195.00)
- HR (>300 Readings)
- Mean Intermittent Device: 83.90 (Range: 46.00 – 161.00)
- Mean Nexfin: 83.81 (Range: 43.00 – 159.00)
- Pearson Correlation Coefficient 0.97
Strengths:
- 1st trial to report comparisons of intermittent BP and HR measurements obtained by standard automated devices vs the Nexfin continuous finger cuff monitoring in patients presenting to the ED
- Final ED diagnoses were broad allowing for some generalizability in the acute setting
- Results of Nexfin monitoring were blinded to the treating ED nurses and ED physicians
Limitations:
- The gold standard used in this study was intermittent BP cuffs and ED monitors. A better gold standard would have been arterial lines.
- Small trial with only 40 patients enrolled, but there were over 300 BP and HR readings for evaluation
- Due to requirement of informed consent the sickest patients were not able to be enrolled in this study
- It is unclear what the effect of severe peripheral vascular disease or peripheral vasoconstriction would have on the accuracy of finger cuff technology
- There was no calibration of devices before the study
- Measurements of BP and HR were made at the same time but in different upper extremities
- There may be a need to change finger cuffs to alternative digits in some patients (<10% of cases) because of some physical discomfort
- Bland-Altman analysis without corrections for repeated values simplified statistical calculations, but the true limits of agreement may be wider than what is reported in this article
Discussion:
- The best correlation of the Nexfin device to standard automated, intermittent devices was for HR
Author Conclusion: “Continuous BP and HR monitoring measured by the Nexfin finger cuff device in this trial showed reasonable agreement when compared with the intermittent values obtained by automated ED equipment. However, theoretically, noninvasive and continuous monitoring of the BP and HR might better reflect underlying hemodynamics than these same measurements obtained intermittently and, thus, could be important in patient management. More study is needed to determine the optimal method of monitoring these parameters.”
Study#2 [6]:
What They Did:
- Multinational, observational study
- Prognostic Hemodynamic Profiling in the Acutely Ill Emergency Department Patient (PREMIUM)
- Enrolled patients with suspected acute heart failure, sepsis, or stroke
- Continuous noninvasive hemodynamic monitoring was done using the Nexfin finger cuff device
- Hemodynamic measurements were averaged for the initial 15 minutes prior to therapeutic interventions and then for the next 2 – 4 hours
Outcomes:
- Measurements of Cardiac Function for AHF, Sepsis, and Stroke
- Hemodynamic predictors of 30-day mortality
Inclusion:
- ≥18 years of age
- Patients thought to have acute heart failure, sepsis, or stroke
- No prior hemodynamic altering therapy
Exclusion:
- Unable to perform informed consent
- Unable to be enrolled within 4 hours of ED arrival
- End Stage Renal Disease (ESRD)
- Pregnancy
- Acute ST-segment elevation myocardial infarction
- Unavailable for 30-day follow up
- DNR status
- Aortic valvular disease
- Transferred from another facility
- Excessive agitation
- Receiving any ongoing intravenous home infusions
- LVADs
Results:
- 514 patients enrolled (4 Excluded due to non-analyzable Nexfin readings)
- 185 (36%) Acute Heart Failure
- 194 (38%) Sepsis
- 131 (26%) Stroke
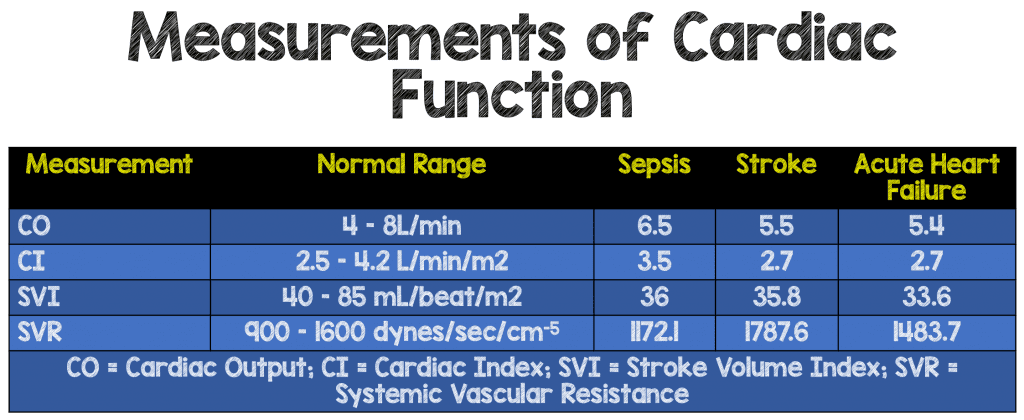
- Measurements of Cardiac Function (CO, CI, SVI, SVR)
- Predictor of 30 Day Mortality:
- SVI was best predictor
- AHF Cut Point: 24.2 (AUC 0.730)
- Sepsis Cut Point 35.3 (AUC 0.687)
- Stroke Cut Point 22.16 (AUC0.741)
- Best Predictor in AHF
- Stroke Volume: Optimal Cut Point 39.91 (AUC 0.783)
- Best Predictor in Sepsis
- Cardiac Output: Optimal Cut Point 5.80 (AUC 0.702)
- Best Predictor in Stroke
- SVI: Optimal Cut Point 22.16 (AUC 0.741)
- SVI was best predictor
Strengths:
- Multicenter study
- Attempted to find hemodynamic variables that may assist in diagnosis and therapeutic interventions that may benefit patients and decrease mortality
Limitations:
- As this was an observational trial, never studied before, no power calculations were completed for enrollment numbers
- No clinical scales or scoring systems were used to further define the severity of disease
- Non-invasive hemodynamic monitoring was not compared to gold standard alternatives (i.e. arterial lines)
- Convenience sample may not be representative of all patients with suspected diseases
- Although cutoff values for 30-day mortality are given, there is significant overlap of hemodynamic variables amongst disease states
- Non-invasive hemodynamic variables responses to treatments over time were not evaluated
Discussion:
- Presenting hemodynamic variables differed significantly amongst patients with suspected AHF, sepsis, and stroke, which could potentially help distinguish one disease state from another when overlapping clinical features are present (i.e. dyspnea)
- Although there are scores that help predict in-hospital mortality, the issue is they don’t provide specific guidance for therapeutic actions, but hemodynamic measurements might help with direct interventions
Author Conclusion: “Presenting ED noninvasive HD data has not been previously reported in any large patient population. Our data suggest a potential role for early noninvasive HD assessments aiding in diagnosing of patients, individualizing therapy based on each person’s unique HD values and predicting 30-day mortality. Further studies and analyses are needed to determine how HD assessments should be best used in the ED.”
Clinical Take Home Point: At this time, finger cuff non-invasive hemodynamic monitoring devices are not ready for primetime. There is no strong evidence that they are accurate in a sick ED population, or that using them affects outcomes of our patients. This just appears to be a very expensive toy, that has not proven that it can do better than what we already do.
References:
- Truijen J et al. Noninvasive Continuous Hemodynamic Monitoring. J Clin Moni Comput 2012; 26: 267 – 278. PMID: 22695821
- Vos JJ et al. Comparison of Continuous Non-Invasive Finger Arterial Pressure Monitoring with Conventional Intermittent Automated Arm Arterial Pressure Measurement in Patients Under General Anesthesia. British Journal of Anesthesia 2014; 113 (1): 67 – 74. PMID: 24740992
- Stens J et al. Non-Invasive Measurements of Pulse Pressure Variation and Stroke Volume Variation in Anesthetized Patients Using the Nexfin Blood Pressure Monitor. J Clin Monit Comput 2016; 30: 587 – 94. PMID: 26318314
- Vos JJ et al. Noninvasive Pulse Pressure Variation and Stroke Volume Variation to Predict Fluid Responsiveness at Multiple Thresholds: A Prospective Observational Study. Can J Anesth 2015; 62: 1153 – 1160. PMID: 26335905
- Nowak RM et al. Noninvasive continuous or Intermittent Blood Pressure and Heart Rate Patient Monitoring in the ED. American Journal of Emergency Medicine 2011; 29: 782 – 789. PMID: 21802881
- Nowak RM et al. Noninvasive Hemodynamic Monitoring in Emergency Patients with Suspected Heart Failure, Sepsis, and Stroke: The Premium Registry. Western Journal of Emergency Medicine 2014; 15 (7): 786 – 94. PMID: 25493119
- The Clearsight System Website
Post Peer Reviewed By: Anand Swaminathan (Twitter: @EMSwami)
The post Is the Future of Non-Invasive Hemodynamic Monitoring Here and Ready for Primetime? appeared first on REBEL EM - Emergency Medicine Blog.
