
 Background: The publication of the MR CLEAN trial in January 2015 changed the face of ischemic stroke care. This was the first study demonstrating a benefit to endovascular treatment of a specific subset of ischemic stroke patients: those with a large vessel occlusion (LVO) presenting within 6 hours of symptom onset. MR CLEAN was followed by a flurry of publications seeking to replicate and refine treatment as well as expand the window for treatment. The REBEL EM team reviewed this literature back in 2018 and, with the help of Dr. Evie Marcolini, created the below workflow:
Background: The publication of the MR CLEAN trial in January 2015 changed the face of ischemic stroke care. This was the first study demonstrating a benefit to endovascular treatment of a specific subset of ischemic stroke patients: those with a large vessel occlusion (LVO) presenting within 6 hours of symptom onset. MR CLEAN was followed by a flurry of publications seeking to replicate and refine treatment as well as expand the window for treatment. The REBEL EM team reviewed this literature back in 2018 and, with the help of Dr. Evie Marcolini, created the below workflow: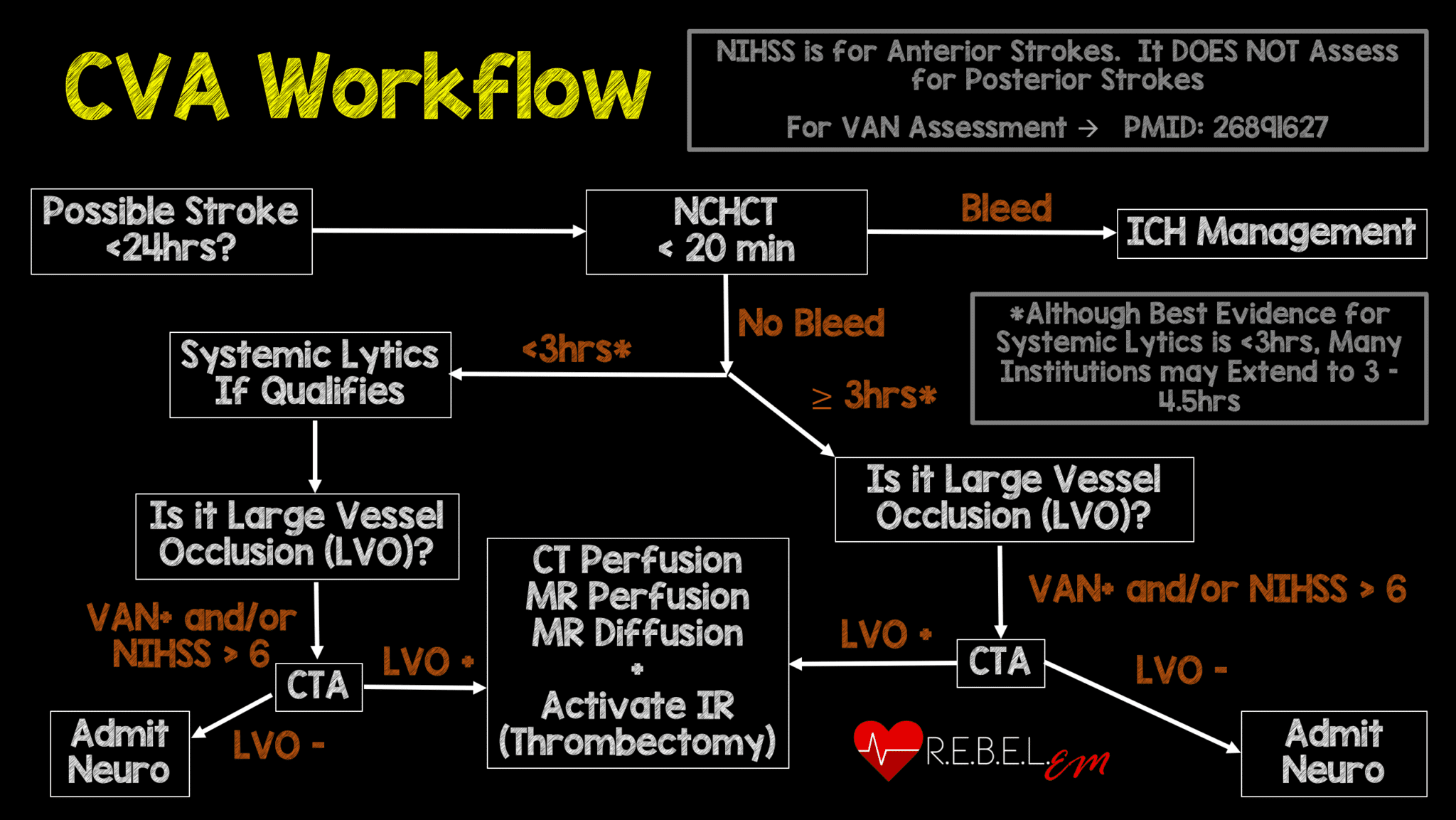
Endovascular therapy has massively changed stroke care but, has not been extensively investigated in patients with large strokes. In fact, most studies have excluded those with larger infarct or ischemic territories focusing on those with Alberta Stroke Program Early Computed Tomographic Score (ASPECTS) > 6 (scale 0-10 with lower score indicating larger infarct burden).
Clinical Question: Does endovascular therapy, in addition to standard medical care, improve clinical outcomes in large strokes in comparison to medical therapy alone?
Article: Yoshimura S et al. Endovascular Therapy for Acute Stroke with a Large Ischemic Region. NEJM 2022; 386(14): 1303-1313. PMID: 35138767
Population: Adult patients with an ischemic stroke with an NIHSS > 6 presenting within 6 hours of time of onset or 6-24 hours from onset with no signal on FLAIR (indicating that infarction is recent) found to have an occlusion of the internal carotid artery or M1 segment of the middle cerebral artery on CT or MR angiography and an ASPECTS score of 3-5.
Outcomes:
- Primary: modified Rankin scale (mRS) assessed at 90 days after randomization
-
Secondary:
- mRS 0-2
- mRS 0-1
- Ordinal shift in mRS at 90 days
- Improvement of at least 8 points on the NIHSS at 48 hours
-
Safety Outcomes
- Symptomatic intracranial hemorrhage along with worsening NIHSS of at least 4 points within 48 hours of randomization
- Any intracranial hemorrhage within 48 hours of randomization
- Death within 90 days
- Recurrent ischemic stroke within 90 days
- Need for decompressive craniotomy within 7 days
Intervention: Endovascular therapy with medical care (alteplase 0.6 mg/kg if indicated)
Control: Medical care alone (alteplase if indicated)
Design: Multicenter, randomized, non-blinded prospective study across 45 hospitals in Japan
Excluded:
- Significant cerebral mass effect with midline shift
- Acute intracranial hemorrhage on CT or MRI
- High-risk of hemorrhage (investigator decision)
- ASPECTS < 3
- Baseline mRS > 1
Primary Results
-
- 235 patients screened across 45 centers in Japan over 35 months.
- 203patients enrolled
- 101 patients assigned to endovascular + medical arm
- 102 patients assigned to medical arm
- 14 patients excluded after randomization
- Received alteplase
- Endovascular arm: 26.7% (27/101)
- Medical arm: 28.4% (23/102)
- Median age = 76 years
- Median NIHSS score = 22
- Median ASPECTS = 3
- Median time from stroke onset to hospital arrival:
- Endovascular Group: 190min
- Medical Care Group: 170min
Critical Findings:
-
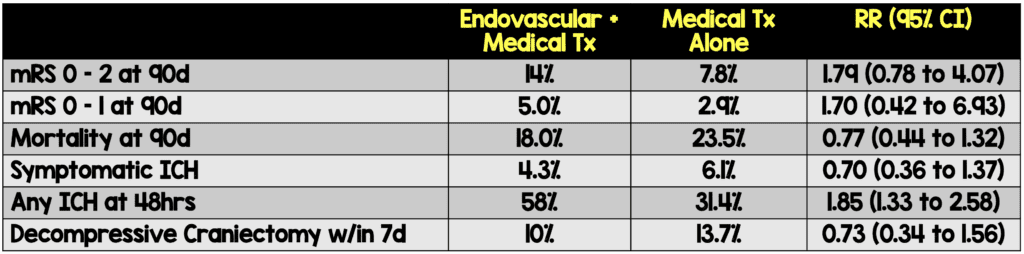
- Primary Outcome (mRS 0-3 at 90 days)
- Endovascular + medical therapy: 31.0%
- Medical therapy alone: 12.7%
- Relative Risk: 2.43 (95% CI 1.35-4.37)
- Secondary Outcomes
- Primary Outcome (mRS 0-3 at 90 days)
Strengths:
- Study asks an important clinical question that has implications for patient outcomes as well as resource utilization
- The study was performed across multiple institutions which increases external validity
- Primary outcome is patient centered (function) as opposed to disease oriented (ie reperfusion)
- Randomization was adequately performed, and baseline characteristics are well balanced
- Researchers used data from an observational registry to construct their statistical analysis and power calculations
Limitations:
- Unblinded study: patients, treating clinicians and outcome assessors were not blinded to treatment arm thus introducing significant bias in the primary outcome assessment as well as some of the safety outcomes (eg symptomatic intracranial hemorrhage)
- Though the mRs is a well-accepted outcome measure, there is some subjectivity to it which threatens the validity of the data
- Though multicentered, the study was only performed in a single country (Japan) thus limiting external validity.
- It is unclear if patients were recruited consecutively or not
- It is unclear how patients were evaluated at 90 days for mRS. Typically, this is done by phone interview which introduces bias.
Discussion:
- Patients in the endovascular arm were much more likely (58% vs 31.4%) to experience intracranial hemorrhage (ICH) within 48 hours.
- The authors reported no difference in symptomatic ICH.
- However, as the clinicians were unblinded, the reporting of symptomatic ICH may be biased.
- Alteplase dose = 0.6 mg/kg (lower than the standard 0.9 mg/kg used in US)
- The lower dose may have resulted in a lower ICH rate than would be seen with the standard dose.
- Over 3 years across 48 sites, only about 200 patients were identified as eligible
- This is important as obtaining CT, CTA/MRA and perfusion imaging, obtaining rapid radiology assessments of imaging as well as ASPECTS and getting patient to neuro-interventional management within a short period of time is challenging and cost intensive.
- Creating an expensive system to provide this care to a small number of patients may not be feasible or cost conscious.
- The researchers included patients who were 6-24 hours out of symptom onset if they had FLAIR imaging that suggested a recent infarct.
- This is interesting as many have commented that we should be moving away from a time threshold for stroke intervention towards an imaging threshold
- For instance, if the patient is 1 hour from onset but has a completed infarction, it’s highly unlikely they’ll benefit from lytics or interventional therapy and, if the patient is 20 hours from onset but has a large ischemic penumbra with minimal infarction they may be more likely to benefit.
Authors Conclusions:
“In a trial conducted in Japan, patients with large cerebral infarctions had better functional outcomes with endovascular therapy than with medical care alone but had more intracranial hemorrhages.”
Our Conclusions: In this small study of patients with large cerebral infarctions with large vessel occlusion, endovascular therapy appears to provide better outcomes than medical therapy alone. However, there is significant bias in this study in terms of outcome evaluation, selection of patients and safety outcome evaluation. Larger studies and external validation of this approach are necessary. The use of imaging instead of purely relying on time from symptom onset to guide management is intriguing.
Potential to Impact Current Practice: There is not enough data to shift current practice in terms of endovascular care for LVO stroke. The decision to offer interventional care will rest with the neurointerventional team. However, we would recommend consulting that service even if the patient has a low ASPECTS score based on this limited data.
For More on This Topic Checkout:
Post Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post RESCUE-Japan LIMIT Trial appeared first on REBEL EM - Emergency Medicine Blog.
