

Background: Could Xuebijing (XBJ) catalyze a paradigm shift in sepsis management? XBJ is an herbal compound used in China to manage various inflammatory and infectious processes in recent years, including sepsis.2-6 It is an herbal preparation comprising a blend of five herbs: Carthami Flos, Paeoniae Radix Rubra, Angelicae Sinensis Radix, and Salviae Miltiorrhizae.2,5,6 Professor Jinda Wang developed it based on traditional Chinese medicine principles to combat infection and inflammation.4 XBJ received approval from the China Food and Drug Administration in 2004,2 although the FDA of the United States has not approved it. This post evaluates the recent “Efficacy of Xuebijing Injection in Patients With Sepsis (EXIT-SEP)” trial.
Article: Liu S, Yao C, Xie J, et al. Effect of an Herbal-Based Injection on 28-Day Mortality in Patients With Sepsis: The EXIT-SEP Randomized Clinical Trial. JAMA Intern Med. 2023;e230780. PMID: 37126332
Clinical Question: In adult patients diagnosed with sepsis, does XBJ reduce 28-day mortality when added to standard care compared to placebo?
What They Did:
- Investigators performed a randomized, double-blind, placebo-controlled, multicenter, parallel-group trial.
- They consecutively enrolled 1,817 ICU patients from 45 sites across China.
- Patients were randomized in a 1:1 fashion.
- 911 patients were randomized to the intervention and 906 to the control.
- Providers were instructed to follow local sepsis management guidelines.
- Trial registered with clinicaltrial.gov: NCT03238742
- The Development Center provided funding for Medical Science and Technology, National Health Commission of the People’s Republic of China.
Population:
Inclusion Criteria:
- Age 18-75 years old
- SOFA score 2-13
-
Admitted to the ICU with a diagnosis of sepsis 3.0,
- “Life-threatening organ dysfunction caused by a dysregulated host response to infection.” 7 Clinically speaking, they used an increase of the SOFA score by 2 or more points to indicate this. This varies from the 2001 definition, which used SIRS criteria and was thought to overly focus on inflammation.7
Exclusion criteria:
- Diagnosed with sepsis for more than 48 hours
- Inability to provide consent
- Severe primary disease (unresectable tumors, hematologic diseases, HIV)
- Severe liver or kidney dysfunction
- Therapy with an immunosuppressant or recent organ transplant
- Pregnancy or breastfeeding
- Participation in another clinical trial within 30 days
Intervention:
- XBJ (100 mL) mixed with 100 mL of normal saline (NS) every 12 hours for 5 days (total volume of 200cc).
Control:
- Placebo: 200 mL of NS q12hr for 5 days
Outcomes:
Primary outcome:
- All-cause mortality at 28 days after randomization
Secondary outcomes:
- ICU and in-hospital mortality
- ICU and hospital length of stay
- Number of ICU-free days at 28 days
- Cumulative ventilator-free days at 28 days
- Change in APACHE II and SOFA scores
Safety Outcomes:
- Reported adverse events
Results:
Enrollment:
- Screened 4692 potential participants from 45 ICUs between the months of October 20, 2017, to June 20, 2019.
-
1,817 enrolled and randomized
- 911 randomized to the XBJ cohort.
- 906 randomized to the Placebo cohort.
-
67 did not receive the intervention to which they were randomized due to the following:
- Death (10)
- Withdrawal of consent (32)
- Later discovery of exclusion criteria at the time of randomization (25)
- 1,325 (75.7%) completed the full treatment period
- Mean Baseline SOFA score was 7.1 in both groups
- Mean APACHE II score was 12 in both groups
- Almost half of the patients had septic shock at enrollment
- Mean time from sepsis identification to randomization was 1.4 days
- 2 most common sites of infection were lung (44.8%) and inta-abdominal (32.1%)
- Majority of infections were community-acquired (≈84%)
- Over half the patients were on mechanical ventilation (≈53%)
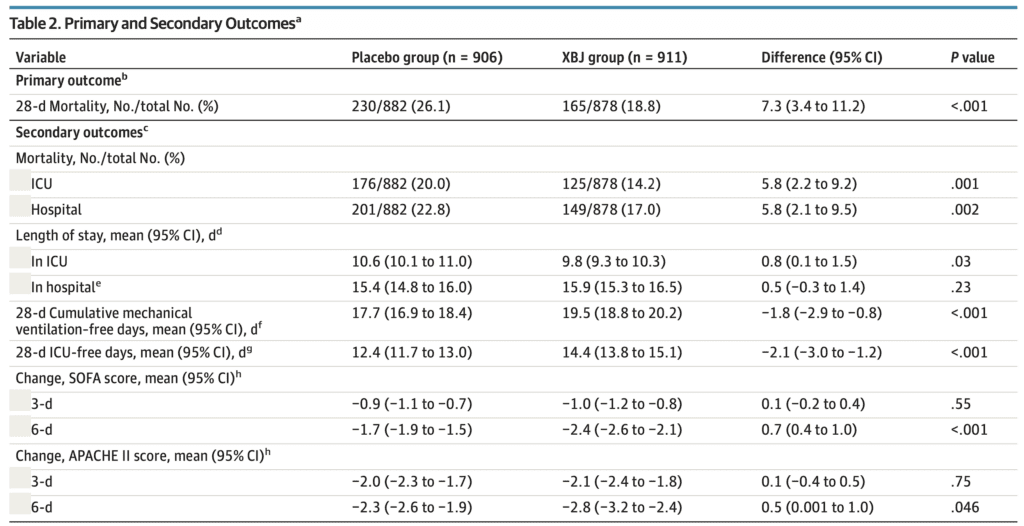
Primary Outcome Results:
-
28-day mortality rate:
- Placebo 26.1% (230/882) vs. XBJ 18.8% (165/878); P<0.001
- The risk difference was 7.3% (95% CI, 3.4-11.2)
- 33 patients in the intervention arm and 24 in the placebo group had unknown mortality status at day 28 and were assumed alive.
Secondary Outcome Results:
-
ICU mortality:
- placebo 20.0% vs. XBJ 14.2%
- The risk difference was 5.8% (95% CI, 2.2 to 9.2, P = .001)
-
Hospital mortality:
- Placebo 22.8% vs. XBJ 17.0%
- The risk difference was 5.8% (95% CI, 2.1 to 9.5, P = .002)
-
ICU-free days:
- Placebo 12.4% vs. XBJ 14.4%
- The risk difference was –2.8% (95% CI, -3.0 to -1.2, P < .001)
-
28-day cumulative mechanical ventilation–free days:
- Placebo 17.7% vs. XBJ 19.5%
- The risk difference was –1.8% (95% CI, -2.9 to -0.8, P < .001)
Safety Outcome Results:
-
Adverse Events:
- 422 patients (24.1%) experienced at least one adverse event
- 200 patients (22.9%) in the XBJ group vs. 222 patients (25.3%) in the placebo group) within 28 days of follow-up.
- No treatments were discontinued due to drug toxicity
-
Elevated transaminases and leukocytosis were most common in the XBJ group
- ALT, 5.8%, AST 4.4%, and WBCs 4.1%
- Similar distributions of adverse events between XBJ and placebo without reported drug-related severe adverse events.
Strengths:
- The double-blind, randomized, placebo-controlled, multicenter trial design limits bias and increases external validity.
- Patients were enrolled consecutively, which limits selection bias.
- The trial was overseen by a blinded steering committee and an independent data and safety monitoring board.
- Investigators registered the trial at clinicaltrials.gov, increasing transparency and reproducibility.
- Investigators asked a patient-centered research question.
- The primary outcome was patient oriented.
- The sample size was large.
- The assumed mortality rate for power calculation was accurate, and the study was appropriately powered.
- Randomization and concealment used a secure web-based system which limits the potential for unblinding.
- Demographics, severity of illness, and comorbidities were well balanced between groups and the intervention and control groups have the same prognosis.
-
Investigators performed an Intention-To-Treat analysis which analyzes patients in groups they were assigned whether or not they followed the study protocol.
- This reflects a real-world clinical practice that maintains randomization and limits bias.
-
Investigators performed multiple sensitivity analyses which confirmed the main results further increasing the credibility and robustness of the data.
- Worse-Case Scenario analysis
- Tipping point analysis
Limitations:
- The composition of XBJ complicates the determination of the specific compounds responsible for observed effects.
- Patient enrollment exclusively from a single country (China) restricts the applicability of findings to diverse global populations.
- The study’s limited inclusion of severely ill patients (APACHE score >25, severe renal or hepatic dysfunction) affects its relevance to sicker patients with a higher risk of comorbid conditions.
- The most common reasons for exclusion included severe liver and kidney dysfunction, severe primary disease, and diagnosis of sepsis ≥48hrs. This limits generalizability of to patients with multiorgan dysfunction, terminal diagnoses, and delayed presentations.
- The study was powered to detect differences in secondary outcomes, but this sould be interpreted cautiously as there were 10 secondary outcomes without adjustments for multiple comparisons (i.e. hypothesis generating).
- Many of the secondary outcomes were changed well after most of the data was acquired.
- Nearly two-thirds of the enrolled population were men, limiting generalizability.
- Conducting the study solely in the ICU limits its transferability to other treatment environments, such as the ED.
- The distribution of patients enrolled across each center remains unspecified, potentially compromising the generalizability of the data if a few centers contributed a substantial number of participants.
- There was a potential for unblinding due to differences in drug appearance, coupled with the awareness of study nurses, and their involvement in patient care remains unclear.
- Administration of antibiotics before randomization introduces uncertainty regarding its impact on the collected data.
- A higher proportion of lost follow-up cases within the XBJ group introduces a bias.
- The predominance of lung and intra-abdominal infections (constituting nearly 80% of cases) limits the broader applicability of XBJ to infections from other sources.
- The lack of long-term follow-up data hinders the understanding of extended treatment outcomes.
- XBJ, an herbal preparation without FDA approval, may pose challenges in countries unfamiliar with alternative/complementary medicine.
Discussion:
Inside the Numbers:
- The number needed to treat (NNT) is a fundamental concept in clinical research.8 It helps translate the impact of treatment into a more understandable and clinically relevant statistic.8 The NNT for XBJ in this paper is 13.7, which indicates that, on average, about 14 patients with sepsis would need to be treated with XBJ to prevent one death in 28 days.
- The Fragility Index measures a clinical trial’s result robustness, especially its statistical significance for the primary outcome. It gauges how outcome changes in the treatment and control groups could alter the study’s findings. A low index indicates fragile conclusions, easily reversed by a few events. A high index reflects robust results, resistant to minor event fluctuations. This paper’s Fragility Index of 29 indicates that changes in the outcome events for 29 patients are needed to alter the statistical significance of the study’s results.
- Thirty-three patients were lost to follow-up, and investigators performed multiple sensitivity analyses on these patients. The sensitivity analysis comprehensively evaluates the study’s outcomes while considering various scenarios and factors that might influence the results. By systematically altering certain variables or assumptions, researchers can assess the robustness and reliability of their findings. This paper’s sensitivity analysis confirmed the initial findings and demonstrated that the study’s conclusions hold even under various conditions, reinforcing the research outcomes’ reliability and credibility. To shift the study’s outcome, nearly all 33 patients lost to follow-up would have to meet the primary outcome within the experimental group.
Perfectly Plausible?:
- The data are exciting and note-worthy. However, It’s essential to remember that the investigators excluded many very ill patients. Therefore, the studied population was relatively healthy to begin the study. Moreover, patients already began antibiotic treatment before they were randomized to receive XBJ or placebo, which may overestimate or alter the findings. Additionally, XBJ contains many compounds whose exact mechanisms are unclear. While it’s possible that some of its constituents are beneficial, it’s unlikely that all components are active against sepsis. The findings are reminiscent of other recent sepsis vitamin C therapies, eventually proven ineffective in follow-up validation studies.
Xuebijing and the FDA
- Although Xuebijing is listed as an herbal supplement used to treat a specific disease, an IV formulation would likely be subjected to the exact approval requirements of any new drugs.9 Specifically, there must be an application for licensure with two studies showing that the drug’s benefits outweigh the risks.9 Occasionally, the FDA will approve a drug based on a single high-quality study that covers a broad range of patients with clear benefits.9 While there is a significant benefit and few adverse effects discussed in this study, it does not demonstrate sufficient external validity to be applied to patients in the ED. As such, for XBJ to be considered for approval by the FDA, it would require an additional study on a broad range of patients, preferably in a US-based or international population more closely representative of the septic patient in the United States.
Authors Conclusions: “In this randomized clinical trial among patients with sepsis, the administration of XBJ reduced 28-day mortality compared with placebo.”
Clinical Bottom Line:
XBJ shows potential in improving survival rates for sepsis patients. However, its composition appears to be a random assortment of herbs with varying doses. While the study is promising, a more precise formulation must be established and verified in further trials before it can be integrated into clinical practice.
- First10EM: Finally, a cure for sepsis: Herbs
- REBEL EM: Sepsis 3.0 Definitions
- REBEL EM: Methylene Blue for Sepsis
- REBEL EM: VITAMINS for Sepsis
References:
- Liu S, Yao C, Xie J, et al. Effect of an Herbal-Based Injection on 28-Day Mortality in Patients With Sepsis: The EXIT-SEP Randomized Clinical Trial. JAMA Intern Med. 2023;e230780. PMID: 37126332
- Song Y, Yao C, Yao Y, Han H, Zhao X, Yu K, Liu L, Xu Y, Liu Z, Zhou Q, Wang Y, Ma Z, Zheng Y, Wu D, Tang Z, Zhang M, Pan S, Chai Y, Song Y, Zhang J, Pan L, Liu Y, Yu H, Yu X, Zhang H, Wang X, Du Z, Wan X, Tang Y, Tian Y, Zhu Y, Wang H, Yan X, Liu Z, Zhang B, Zhong N, Shang H, Bai C. XueBiJing Injection Versus Placebo for Critically Ill Patients With Severe Community-Acquired Pneumonia: A Randomized Controlled Trial. Crit Care Med. 2019 Sep;47(9):e735-e743. PMID: 31162191
- Zheng J, Xiang X, Xiao B, Li H, Gong X, Yao S, Yuan T. Xuebijing combined with ulinastation benefits patients with sepsis: A meta-analysis. Am J Emerg Med. 2018 Mar;36(3):480-487. doi: 10.1016/j.ajem.2017.12.007. Epub 2018 Jan 16. PMID: 29373169.
- Han D, Wang R, Yu Y, Sun M, Teschke R, Romeiro FG, Mancuso A, Song T, Peng Z, Han B, Zhou X, Bao W, Li Q, Zheng K, Li Y, Bai Z, Guo X, Qi X. Xuebijing Injection Combined with Antibiotics for the Treatment of Spontaneous Bacterial Peritonitis in Liver Cirrhosis: A Meta-Analysis. Evid Based Complement Alternat Med. 2018 Mar 19;2018:2989846. PMID: 29743922
- Chen H, Bai Z, Li H, Wu Y, Yao H, Wang L, Lin H, Tong Z, Teschke R, Qi X. Efficacy of Xuebijing Injection for Acute Pancreatitis: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Evid Based Complement Alternat Med. 2021 Apr 26;2021:6621368. PMID: 34221082
- Lv J, Guo X, Zhao H, Zhou G, An Y. Xuebijing Administration Alleviates Pulmonary Endothelial Inflammation and Coagulation Dysregulation in the Early Phase of Sepsis in Rats. J Clin Med. 2022 Nov 11;11(22):6696. PMID: 36431172
- Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, Bellomo R, Bernard GR, Chiche JD, Coopersmith CM, Hotchkiss RS, Levy MM, Marshall JC, Martin GS, Opal SM, Rubenfeld GD, van der Poll T, Vincent JL, Angus DC. The Third International Consensus Definitions for Sepsis and Septic Shock (Sepsis-3). JAMA. 2016 Feb 23;315(8):801-10. PMID: 26903338
Guest Post By:

Lynnsey Moss, MD
PGY-3, Emergency Medicine Chief Resident
Vassar Brothers Hospital, Poughkeepsie, New York
Email:lynnsey.moss@nuvancehealth.org

Marco Propersi, DO FAAEM
Vice-Chair of Emergency Medicine
Assistant Emergency Medicine Residency Program Director
Vassar Brothers Hospital, Poughkeepsie, New York
Twitter: @marco_propersi
Post-Peer Reviewed By: Salim R. Rezaie, MD (Twitter: @srrezaie)
The post An Herbal Hope: Is XBJ A Game-Changer in Sepsis Management? appeared first on REBEL EM - Emergency Medicine Blog.
